Role of progression free survival in the approval process and European HTA assessments
Posterbeitrag
Zusammenfassung (nur auf Englisch verfügbar)
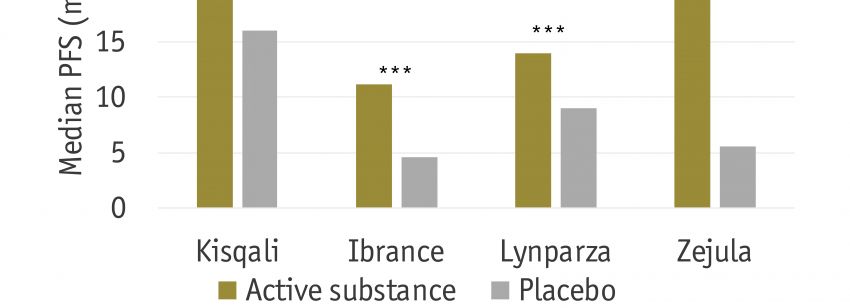
Most cancers are characterized by a progressive course of disease, ultimately leading to the death of the patients. Thus, in the medical world, prevention of the disease progress, measured by progression free survival (PFS), is considered a core parameter with a clear patient relevant benefit. This is reflected in a large fraction of clinical studies in cancer in which PFS serves as the primary endpoint, and thus determines the success of the treatment. While the European Medicines Agency (EMA) considers PFS to be an important factor in assessing the efficacy of a new drug, HTA agencies such as G-BA, NICE and HAS are divided. The aim of this research is to analyze previous and current assessments of PFS by European HTA agencies and to compare them to the assessment by EMA.
Download
Hier stellen wir Ihnen unsere englisches Poster als kostenlosen Download zur Verfügung.