Digital Health Applications (DiGA) & Digital Pharma
The Challenge
Potentials and challenges of the digital health market
The introduction of the Digital Care Act (DVG - „Digitale-Versorgungs-Gesetz") in 2020 has led to an significant spurt in growth of digital health applications. As a result, digital health applications are receiving increased attention in the German health system. Among all stakeholders, the advantages of digital health applications are emphasized and a large market potential is attributed to enhance health care in a targeted manner with regard to efficiency and quality. Increasing numbers of new manufacturers are entering the digital healthcare market, while established pharmaceutical and medical-technology companies are expanding their product portfolio with digital applications and services.
Revolution in the assessment of eligibility for reimbursement
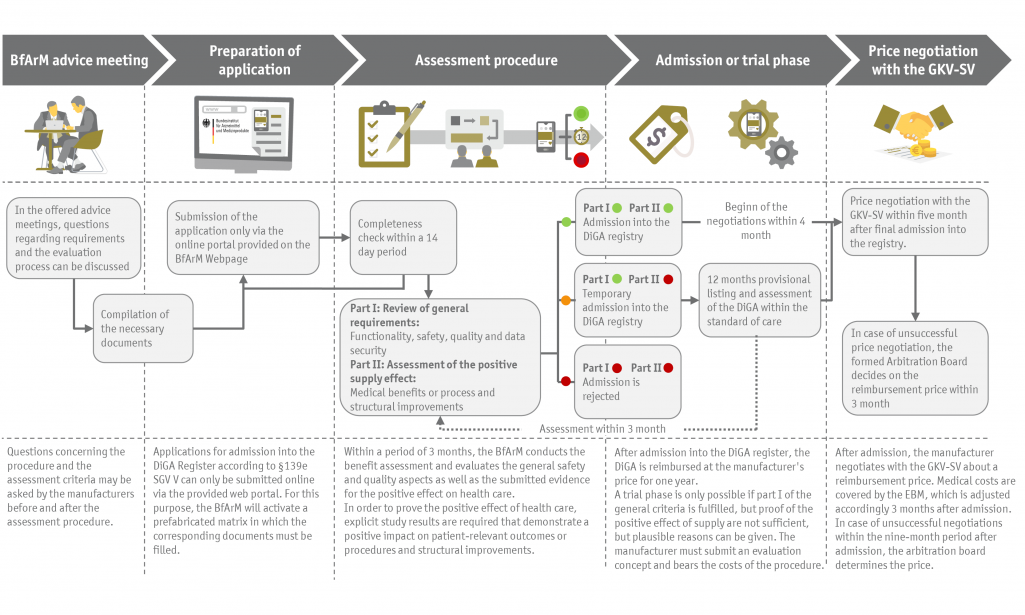
The Digital Supply Act - DVG with the new "fast-track procedure" revolutionizes the assessment of the benefit and the reimbursability of digital innovations. For the first time, the competence for assessment does not lie in the hands of a body of joint self-government, but in those of a federal authority, the Federal Office for Drugs and Medical Devices - BfArM. Previously lengthy procedures can thus be circumvented which leads to a significantly faster attainment of eligibility for reimbursement. The evaluation period is limited to three months in the fast-track procedure, within the framework of the so-called application for inclusion in the DiGA registry according to § 139 SGB V.
Faster application procedure, but regulatory requirements remain on a high level
Although the assessment process has been significantly shortened, DiGA manufacturers should not underestimate the new strategic challenges. In particular, the criteria according to which a digital health solution can also be classified as a DiGA are very restrictive and must be verified in detail. Likewise, comprehensive evidence for technical safety and quality must be provided, as well as profound evidence for the positive effect on care provision, which explicitly requires clinical trails.
Manufacturers of digital innovations should address the requirements of the process as early as possible and consider the following strategic issues:
- How does my product meet the criteria of a digital health application?
- Which regulatory requirements apply to a DiGA, which technical and which medical criteria must be fulfilled?
- How do I argue the positive effect on care provision and which "effects" are included? How does my DiGA fit into the current care context?
- What evidence must be provided for the positive effect on care provision and is my existing study evidence sufficient?
- What does the fast-track pilot regulation mean and what are the involved requirements and costs?
- Which price mechanisms is my digital health care application subject to in the German market and how can I define a strategic price (market introduction price) for my product?
- Which requirements do I have to meet when negotiating prices with the GKV-SV according to §134 SGB V? How can I optimally argue the added value of my DiGA in the form of an appropriate price?
The solution
Our approach
Benefiting from our profund reimbursement expertise
With the DVG and the fast-track procedure, a new reimbursement option for digital health applications has been established. However, the assessment methodology is not fundamentally new, as it is based on already known elements from the AMNOG Assessment and the pilot regulation, which are now combined. Due to our broad experience in numerous projects in the entire field of market access strategies and benefit assessments, we are able to leverage on our expertise and advise clients on the new strategic challees.
-
Market access and reimbursement strategy
In cooperation with our clients, we develop tailor-made market access strategies that include the entire context of the market access process. Our diverse expertise enables us to take on different perspectives of regulatory stakeholders and to identify and anticipate problems and hurdles in advance.
-
Risk assessment and support for advice meetings with the BfArM according to § 23 DiGA-V
In systematic risk analyses, we identify and evaluate possible roadblocks in the market access process. Furthermore, we provide support and guidance in the preparation of consulting meetings with the BfArM according to §23 DiGA-V, in which the course for the subsequent benefit assessment can already be set.
-
Cost-effectiveness assessments
Preparation of structured and stakeholder-specific cost-benefit evaluation (incl. Excel modelling) to assess and demonstrate the economic benefits and cost-effectiveness of medical products.
-
Preparation of applications for inclusion in the DiGA directory according to §139e
Advice and support throughout the entire preparational phase of the application procedure for the admission into the DiGA register according to §139e SGB V. We review your application documents regarding the probability of success or compile convincing application documents, if requested.
-
Evaluation of evidence and proof of positive effects on care provision
Even if the evaluation procedure leads to a considerable acceleration, the requirements for the necessary proof of studies and justification of the positive supply effect should not be underestimated. Concerning your current clinical evidence, we advise you regarding the reliability and the development of a convincing value argumentation.
-
Strategic pricing
With the DVG, the implications for the pricing of DiGAs have fundamentaly changed. Learn more about our consulting services on strategic pricing here.
-
Price negotiation with the GKV-SV according to §134 SGB V
The method of negotiating reimbursement prices is completely new for manufacturers outside the pharmaceutical sector. We offer assistance in development of a price and negotiation strategy and guidance you throughout the entire negotiation process, including any arbitration proceedings that may be necessary.
-
Development of alternative reimbursement options and reimbursement roadmaps
If your product is not suitable for the Fast Track and does not meet the criteria of a DiGA, we can examine alternative reimbursement possibilities within the framework of social legislation. Find more about our consulting services for the market access of medical devices and reimbursement strategies here.
Get in touch

Gründerin und Geschäftsführerin
Fax: +49 511 64 68 14 18