Orphan drugs in Germany
Orphan drugs and the specific market access challenges are at the core of our expertise.
For the market access of orphan drugs in Germany, there are specific challenges that the manufacturer faces due to the rarity of the disease, like small population size and studies of lower evidence levels. As a result, there is a considerable uncertainty about the added value of the drug which can lead to intense and sometimes strenuous negotiations with high rebates. Pharmaceutical companies planning to introduce new orphan drugs into the German market should prepare a sound strategy well in advance to ensure a successful market entry.
SKC consulting is a strategic consultancy focussed on the increasingly challenging market access environment of innovative drug products. We support the successful market access both on a strategic and an operational level.
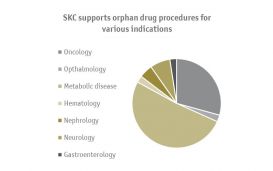
As market access special forces, the strategic challenges of orphan diseases, ATMPs, repurposed drugs and products altering the treatment paradigm are our specialty. We are involved in every 5th orphan drug AMNOG procedure (52 of a total of 248 AMNOG procedures, as of May 2023).
Orphan drugs are a core topic of our publications and white papers. Further, we are producing and publishing our SKC podcast series "The Profcast - rare diseases and their therapies", that aims to address the special circumstances of patients with rare diseases, the associated medicine and the challenges for research, development, economics and reimbursement.Please find a selection of our recent publications below.
Our mission

Whitepaper, publications and blogs

AMNOG orphan tracker:
In our orphan tracker, we examine ongoing AMNOG procedures for orphan drugs.
Publication (2023):
The Pathology of Niemann-Pick Type C in Pediatric and Adult Patients

SKC podcast (2022/23):

Blog (2022):

SKC Whitepaper (2021)
Orphan drugs in Germany - Lessons learned from 10 years AMNOG, best and worst practices

Publication (2021):

SKC Whitepaper (2020)
Registry requirements for the German Benefit Assessment of pharmaceutical products
SKC Whitepaper (2020)

Publication (2019)
Publication (2018)
Orphan drugs' market access challenges in Europe from a German perspective