Trial regulations: strategic support (pursuant to §137e SGB V)
Client: Swedish medicaltechnology company, operating throughout Europe
Field of application: Asthma diagnostics
Challenge
The company has been marketing its products in Germany for more than 10 years. Although the medical specialist market share was covered to 80% through individual health services and private health insurances, the reimbursement through statutory health insurance in the outpatient sector, especially with general practitioners, did not exist.
SKC was assigned to examine suitable reimbursement pathways and to develop a promising approach. In the further course of the project the main focus lied on the application for the probatory regulation according to §137eSGB V which was implemented following the first application to strategically support the process and to implement a flanking stakeholder management.
Solution and approach
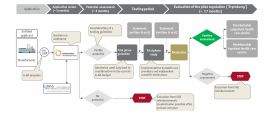
First the medical care situation was screened and product's value proposition (the value story) was analyzed. Subsequently the main access routes were evaluated and the trial regulation pursuant to §137e SBG V was identified as a potential pathway.
The strategic decision to pursue in this direction was prepared and made at the Swedish headquarters.
What really counts during the probation period is the preparation of the audit request in the anticipation of the key decision parameters as well as the preparation of the study.
- For this purpose, the first step was to define the value story with key messages for all stakeholders.
- Then, the next step was to define the basic strategic logic for the dossier, to support the dossier creation and to prepare and implement the stakeholder management.
Added value
The proposal for the trial was positively evaluated, which means that the product was granted a benefit potential. The procedure was selected for two areas of application. This success is not self evident, since the G-BA only decided positively on four out of 50 proposals.
The prices for the SHI-reimbursement were negotiated within the scope of a two-year study period and the product was incorporated into clinical care in accordance to the conditions of § 137e SGB V and the associated rules of procedure.
