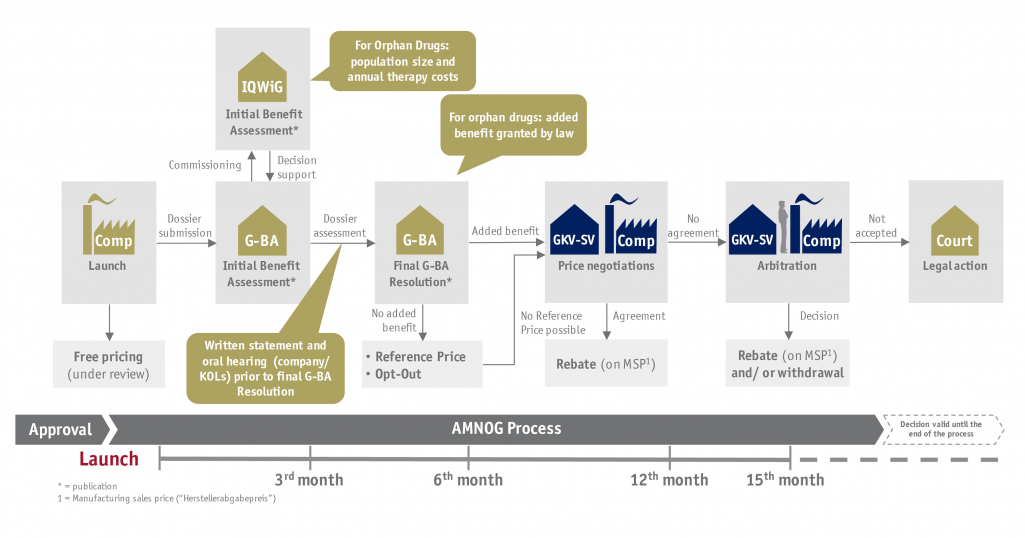
Benefit Assessment (AMNOG, medical devices)
The challenge
Everything in context
Most markets now take their reimbursement decisions for innovative health technologies as part of an HTA. In Germany the AMNOG (Act on the Reform of the Market for Medicinal Products) forms the basis of the market access for innovative pharmaceuticals. But also new examination and treatment methods based on medical devices are confronted with a benefit assessment under certain conditions. In the worst case, the method may be excluded from the service package of the statutory health insurance.
In the context of the benefit assessment, medical products must prove an (added) benefit compared to an appropriately comparative therapy. This (additional) benefit of a clinical method must specifically be presented in a value dossier from the company. To do so, the following core questions can be of assistance:
- How can the first consultation with the Joint Federal Committee (G-BA) and the statement procedure be utilized optimally?
- How can the value of the product for the German market be determined and emphasized in the best possible manner?
The solution
Consider market access early
Due to the growing global relevance of HTAs, we urge medication and medical device manufacturers to develop holistic market access strategies at the same time as regulatory processes, or better by the time clinical trials are initiated.
Hypotheses on market developments must be established early in order to be optimally prepared in the subsequent process because of the high market volatility and rapidly changing conditions.
Communicate purposefully
In most countries, the focus of HTAs is often the value dossier that is part of the assessment process. It is not only important to prove methodological and technical accuracy using the correct format and the correct representation of the data, equally important are communicative abilities and strategic prudence. This allows companies to address different stakeholders inside and outside the company in a targeted manner.
Our appraoch
Comprehensive expertise
We know the external decision makers and multipliers so that relevant arguments from a value story can be extracted for each stakeholder group. In the sense of the reporting strategy, our team of experienced medical writers and scientists create complete value dossiers or select segments thereof. This eliminates interfaces and coordination requirements with different service providers. We also have an all-encompassing perspective: We consider both the requirements of other HTA authorities (e.g. NICE, HAS) as well as alternative sales and access routes, like those of social law and sub-legislative regulations.
-
Strategy development
Development of comprehensive market access/reimbursement/reporting strategies for German and European reimbursement
-
Monitoring
Compilation of all information and scientific findings on the medical technology method for the G-BA
-
Stress test
Creation of stress tests for consultation requests and reports
-
Value dossier
Creation of value dossiers or individual modules
-
Prepared for G-BA
Creation of consultation requirements and preparation for consultation via G-BA (consultation requirements)
-
Proposal writing
Support for testing proposals according to § 137e SGB V (literature research, study summaries, etc.)
-
Coaching in the assessment process
Guidance through the entire assessment process: Creation of objection handling and Q&A documents, creation of the written statement, preparation of the hearing team, strategic guidance on price negotiations
-
Strategic Pricing
Define balanced prices in a complex reference price system. Read more about this here.
-
Stakeholder and public affairs management
Identify relevant stakeholders and use them efficiently
Get in touch

Founder and Managing Director
Fax: +49 511 64 68 14 18