Full Market Access
The challenge
Early, long-term and overarching vision
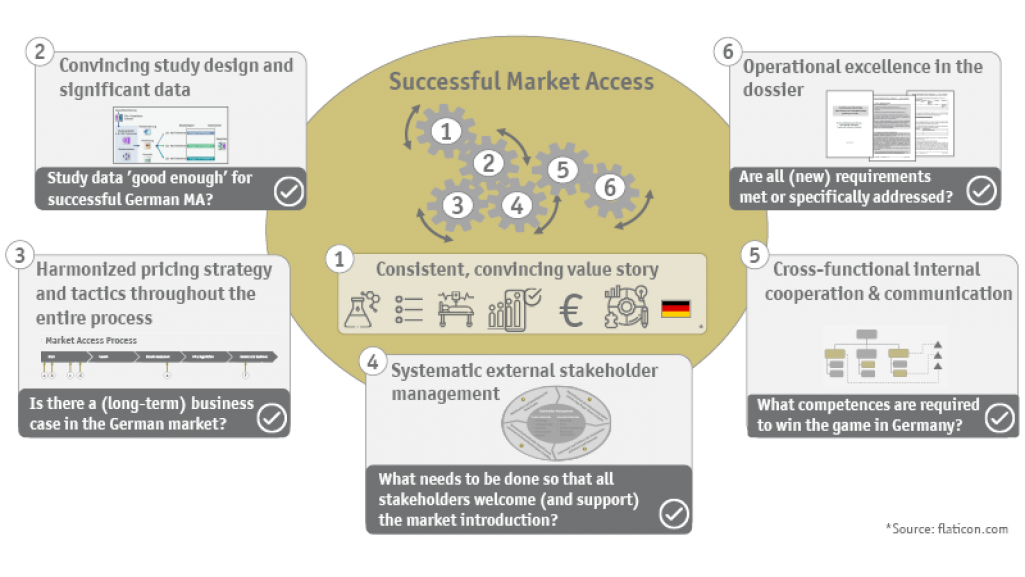
Market access in Germany is a multi-layered and complex process with various obstacles and strict requirements. For a successful business case, the mandatory and optional (or supportive) workstreams must be optimally orchestrated, including: the early goal-oriented development of the overall strategy, the well-assessed re-generation of evidence, the derivation of the respective strategy and tactics for the dossier development and the subsequent price negotiation, the mobilization of the market through a trustful stakeholder management also beyond the AMNOG procedure at the Federal Joint Committee (G-BA) as well as the initiation and continuation of the entire communication with the German HTA authorities.
- Does a business case exist? What reimbursed price seems realistic given the current evidence, the existing standard of care, the addressed therapeutic need, and the size of the population? What sales and price potentials can be generated with the innovation? What is the future shape of the market?
- Who are the relevant stakeholders in Germany, what perspectives do they represent, and how can they be optimally integrated in the various phases?
- Which aspects specific to Germany must be considered when preparing the benefit dossier? What does the benefit dossier have to achieve in order to prove itself in the subsequent AMNOG procedure (statement, hearing, price negotiation, arbitration proceedings)? How can we react flexibly to changing conditions during the preparation of the dossier and still ensure a comprehensive and timely submission?
- How to design a European market access strategy in relation to the currently ongoing implementation of the EU-HTA that takes into account the various hurdles of national HTAs?
The solution
Anticipate criticism, develop awareness & prepare solutions
The German system is clearly structured and transparent but underlies high qualitative and quantitative requirements. The pharmaceutical entrepreneur (pU) often cannot meet these strict and evolving standards due to various possible reasons. It is crucial to be aware of one's own weaknesses and to anticipate methodological as well as content-related points of criticism early on in order to develop solutions. Arrogance should be strictly avoided. Industry-specific knowledge, medical/technical expertise and know-how about economic and political trends are crucial for assessing opportunities and risks.
Excellent, multi-perspective benefit dossier
The heart of market access lies in the so-called benefit dossier - the authoritative basis for the G-BA's decision on the (commercial) future of the drug. In five modules, the clinical, therapeutic, epidemiological and commercial situation is presented to the German HTA authorities in an evidence-based manner. Not only the own value story based on convincing arguments or solid and appropriate evidence, but also the opinions of the numerous different stakeholders have to be adequately considered and consistently combined in the benefit dossier. It is essential to win advocates from all relevant sectors: Stakeholder management is particularly significant for the AMNOG process.
Our approach
Benefit from experience and stay up-to-date
Based on our extensive and continuously growing experience in full market access as well as in numerous individual projects along the value chain (especially with AMNOG processes), we can find the optimal solution for any situation. We take advantage of the sometimes conflicting perspectives of the various stakeholders (e.g. Federal Ministry of Health, G-BA, German National Association of Statutory Health Insurance (GKV-SV), Institute for Hospital Fee Systems (InEK), Institute for Quality and Efficiency in Health Care (IQWiG), etc.) in order to be able to profoundly analyze and understand any issues and test them for potential influencing factors or implications of decision-making.
Strategic vision
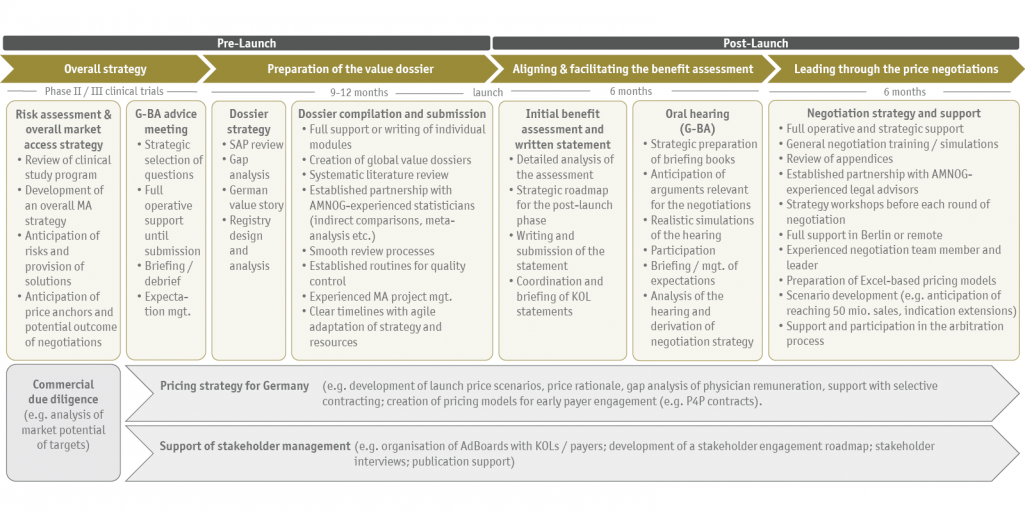
Coupled with our strategic foresight, we enable our clients to bring their ideas to market in a targeted manner or to find alternative paths at an early stage. SKC consulting is not only established for the operational support in the process, but especially for those "tricky cases" in which the strategic component in an already complex system becomes even more important. We structure and control the entire market access process to not only introduce a new product to the market (market access), but also to ensure its long-term (commercial) success (market penetration).
Overall support under one roof
Ideally, we accompany our clients at an early stage and support the planning of phase II/III studies from a market access perspective. However, we often step in at a later stage and develop the optimal market access strategy together with our clients based on a comprehensive risk assessment. Depending on the needs, we can represent the full market access team in Germany for the client: we are not only responsible for the preparation of all important documents (G-BA consultation request, dossier, statement, etc.), but are also part of the team in the oral hearing, the consultation and the price negotiation. You receive the full market access support from us!
Overview of further services:
-
Value Dossier
With our agile value dossier, you receive an evidence-based value line of argumentation that strategically and logically incorporates the product's value story into the entire document. >> Value Dossier
-
Market Access Strategy
We can build your sound market access strategy at an early stage, giving you and all parties involved valuable orientation throughout the entire market access process. >> Market Access Strategy
-
Benefit Assessment (AMNOG, medical devices)
Master with us the complex benefit assessment process so that your innovations enter the market successfully. >> Benefit Assessment
-
Strategic Pricing & Pricing Negotiations
Gain confidence for your decisions with us! We analyze with you individual pricing mechanisms and the European reference price system. >> Strategic Pricing & Pricing Negotiations
-
Stakeholder Management
Gain deep knowledge and a comprehensive understanding of your market and its participants. Incorporate the different stakeholders early and communicate your value messages for profit. >> Stakeholder Management
-
EU-HTA: European Health Technology Assessment
Achieve planning security: With us you can align your projects and your resource planning comprehensively and at an early stage on the EU-HTA and its implications for market access. >> EU-HTA: European Health Technology Assessment
Get in touch

Founder and Managing Director
Fax: +49 511 64 68 14 18