Digital Health Provider
The Challenge
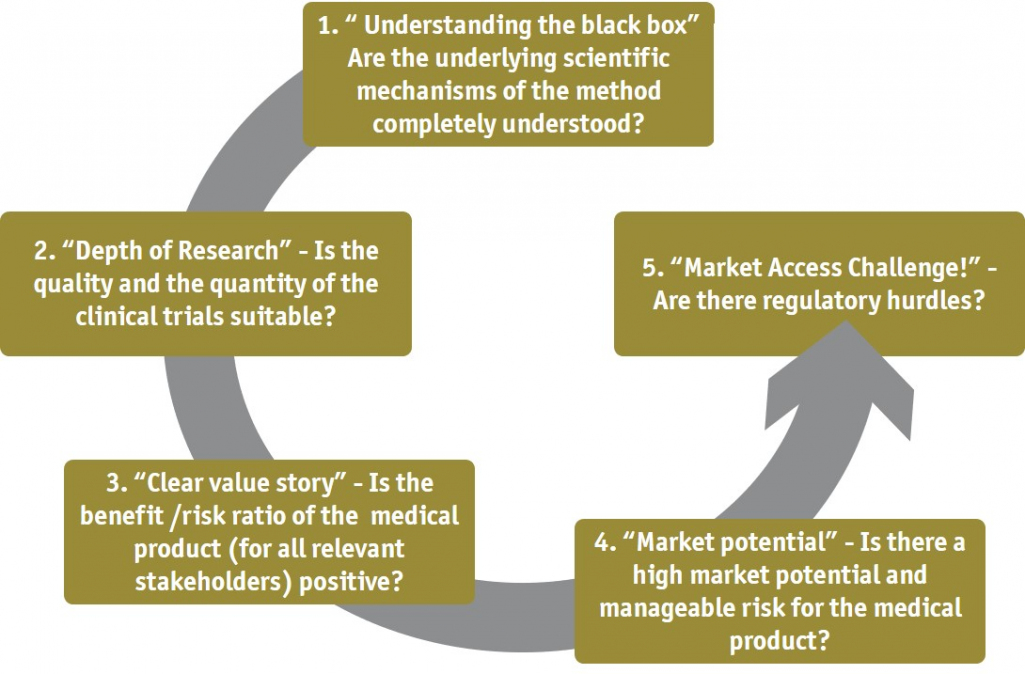
High market potential, high uncertainty and high innovation pressure
Digital innovations and so-called digital health applications (DiGA) are increasingly becoming established as a new pillar in health care provision. New, young, highly innovative and agile companies are emerging, which are developing targeted digitalized health care products (e.g. health apps) to complement the German health care market. Pharmaceutical companies are also subject to this trend. For this reason, they must develop their classic business models and will have to abandon their role as sole providers of pharmaceutical products ("future digital pharma").
According to the chosen perspective, different strategic questions arise, which manufacturers of innovative digital applications or M&A interested parties need to address in regards to the development of portfolio extension by digital innovations:
- How can health technologies be successfully introduced into the German market? What are the specific regulatory and reimbursement requirements?
- What are the requirements in product development and what are the essential criteria to be met for a digital solution to be reimbursable?
- How does my current product concept meet the reimbursement criteria of a Digital Health Application (DiGA)?
- How will patients or the healthcare professionals use my digital product and how can I credibly prove and (considering the possible reimbursement options) successfully demonstrate an added benefit?
- How can the viabilty of start-ups as well as the quality, maturity and market potential of digital applications be realistically assessed, and which start-ups can be considered in terms of cooperation or acquisition?
- How can new digital products be integrated into existing healthcare solutions?
The solution
Strategic Market Access Planning
While new digital products are increasingly being developed, the benefits and thus the question of reimbursement are often largely unresolved or unknown. At the same time, digital health applications and their underlying software are subject to shorter innovation and production cycles, which requires an early planning of effectiveness studies and the consideration of market access challenges.
Development of the conditions for the product viability ("viability check")
A systematic questioning grid is used to approach the question of the maturity and marketability of a digital product. Together, we analyze the relevant opportunities and challenges of reimbursement and define a strategy.
In addition, we support you in mandatory negotiations with statutory health insurances or the National Association of Statutory Health Insurance Funds ("GKV-SV") and other payers and regulatory stakeholders in the health care system when it comes to implementing reimbursement strategies. Should you also require support in the application process or the development of the efficacy study (pilot regulation according to §137e SGB V) we can assist you with our many years of experience.
Our Approach
Upgrade for digital-healthcare-products
Whether you are a startup, global player, service provider, or statutory health insurance provider, SKC supports strategic development and market and reimbursement access by anticipating and integrating all relevant perspectives.
-
Market access und reimbursement strategy
Designing tailored market access strategies specifically for digital innovations, covering the entire context of the market access process.
-
Reimbursement options and roadmap development
Identifying potential pathways into reimbursement and/or evaluating reimbursement options (including stress test of substantive and formal requirements and gap analyses). Prioritization of promising reimbursement options based on reimbursement roadmaps.
-
Development of study designs for benefit assessments of digital innovation
Development of convincing study designs specifically for the proof of benefit of digital innovations.
-
Application for inclusion in the DiGA register according to §139e SGB V
Support and advice throughout the entire phase of application preparation and benefit assessment of digital health applications in the so-called "fast-track procedure" of the BfArM. Preparation of the application for inclusion in the DiGA register according to §139e SGB V.
-
Markt and portfolio analyses
Conducting market and portfolio analyses regarding strategic additions to digital innovations.
-
Commercial Due Dilligence
Assessment and rating of potential target companies from a market and competitive perspective. Matchmaking with potential candidates according to portfolio analyses.
Get in touch

Gründerin und Geschäftsführerin
Fax: +49 511 64 68 14 18