Medtech
The Challenge
The path to reimbursement
Although there are numerous political efforts to unify and standardize reimbursement processes at a European level, the reimbursement of medical products is still solely based on national regulations. In comparison to pharmaceuticals, medical technologies have multiple ways of achieving reimbursement in Germany. However, they differ both largely in their contentual and formal requirements, as well as in their success and market potential.
To successfully master the market access process, companies in the medical technology and biotechnology sector are advised to ask themselves the following questions:
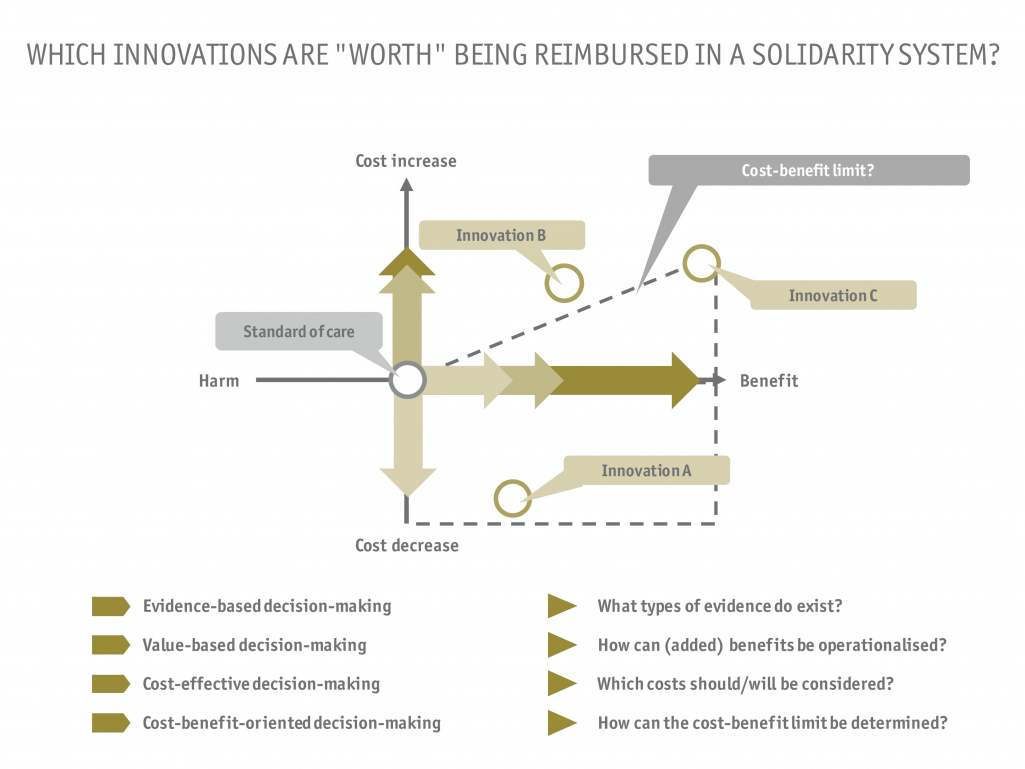
- How must I classify my product or software, and which strategic challenges await me during market access?
- What is the current care situation in Germany and how does my medical device fit into existing healthcare pathways?
- Which regulatory requirements are tied to the clinical application (e.g. in the outpatient sector in comparison to the inpatient sector), and which reimbursement options are suitable?
- Does my current clinical evidence meet the requirements of a benefit assessment procedure and what evidence is required to demonstrate a benefit?
- How can the potential benefits of my medical device be optimally emphasized in the clinical care reality?
- How does the selection of the reimbursement pathway and the current standard of care affect my pricing?
The solution
Target-oriented from the start
A key factor for achieving successful reimbursement is the development of a comprehensive market access strategy starting already in the product development phase. Therefore, knowledge of the regulatory requirements is paramount. How the reimbursement process is structured in detail also depends on the mode of action and the area of application, and eventually also on the defined risk class, which may facilitate or intensify the regulatory requirements. Apart from the chosen reimbursement pathway, a thorough understanding of the market and the care provision is crucial. Only those who know the specific supply path and the stakeholders involved can successfully market a product with a convincing value story that satisfies customer requirements. In order to be able to mobilize the market at an early stage and make use of market potentials, it is important to evaluate the different strategic options in detail with regard to the implementation potential and possible barriers in the market access process.
Our Approach
With experience and knowledge
SKC has extensive experience with reimbursement mechanisms, particulary in Germany, which is the largest market for medical devices in the EU. In addition, due to our longstanding years of activity in the health care sector, we know and understand the key regulatory institutions and are thus able to incorporate their perspective, such as
- the German Federal Ministry of Health,
- the German National Association of Statutory Health Insurance Funds (GKV-SV),
- the German Federal Joint Committed (G-BA), and
- the Institute for the Hospital Remuneration System (InEK).
We jointly develop market access strategies or provide modular support for individual process steps, depending on the objectives of our clients: starting with the creation of a "reimbursement roadmap", to evaluating reimbursement options as well as the development of a product-specific value story, all the way to the analysis of methods, we individual services. Moreover, offer support in necessary negotiations with statutory health insurances or the statutory health insurance scheme and other cost bearers and actors in the health care system in terms of implementing the reimbursement strategy.
-
Market access strategy
Development of holistic product-related market access strategies for the German or European market. Providing a general overview over the German reimbursement system and identifying the steps required for reimbursement qualification in Germany.
-
Reimbursement strategy and reimbursement roadmaps
Identifying potential pathways into reimbursement and/or evaluating reimbursement options (including stress test of substantive and formal requirements and gap analyses). Prioritization of promising reimbursement options based on reimbursement roadmaps.
-
Value Story
Developing a product-specific value story as the basis for argumentation with respect to the various interest groups.
-
Support in benefit and method assessment procedures
Consultancy and support in the procedures of benefit and method assessments (e.g. the assessment of new examination and treatment methods ("NUB-Assessment") according to §135 SGB V, the preparation of applications and preparation of study designs for the testing of new examination and treatment methods ("Erprobungsregel") according to §137e SGB V or the evaluation of new assessment of treatment methods with medical devices of high risk class according to § 137h SGB V).
-
Proof of concept for products in the context of the health care supply reality
Definition of the prerequisites for marketability in a "proof of concept", if required, support for further business model development and definition of business cases, customer and patient journeys and use cases.
-
Negotiation support
Support during hearings with regulatory institutions (especially the Federal Joint Committee - G-BA or the Federal Office for Consumer Protection in consultation procedures - BfArM) or negotiation support for individual contract negotiations with the National Association of Statutory Health Insurance Funds - GKV-SV.
-
Strategic pricing
Definition of market introduction prices and pricing models in view of reimbursement possibilities and the current standard of care.
-
Support with OPS & NUB remuneration applications
Strategic planning and operational support in applications for Operation and Procedure Codes (OPS), NUB application procedures for case-related remuneration acc. § 4 Abs. 2 KHEntgG, as well as in applications for the inclusion of products in the list of medical aids register.
-
Market analysis and market mobilization
Conduction of market analyses and analyses of current care pathways and provision gaps as preparation for strategic product positioning. Landscaping of strategically important stakeholders and mobilization of the market by developing an individual action and communication plan.
Get in touch

Founder and Managing Director
Fax: +49 511 64 68 14 18