Pharma & Biotech
The Challenge
Reimbursement for pharmaceutical innovations
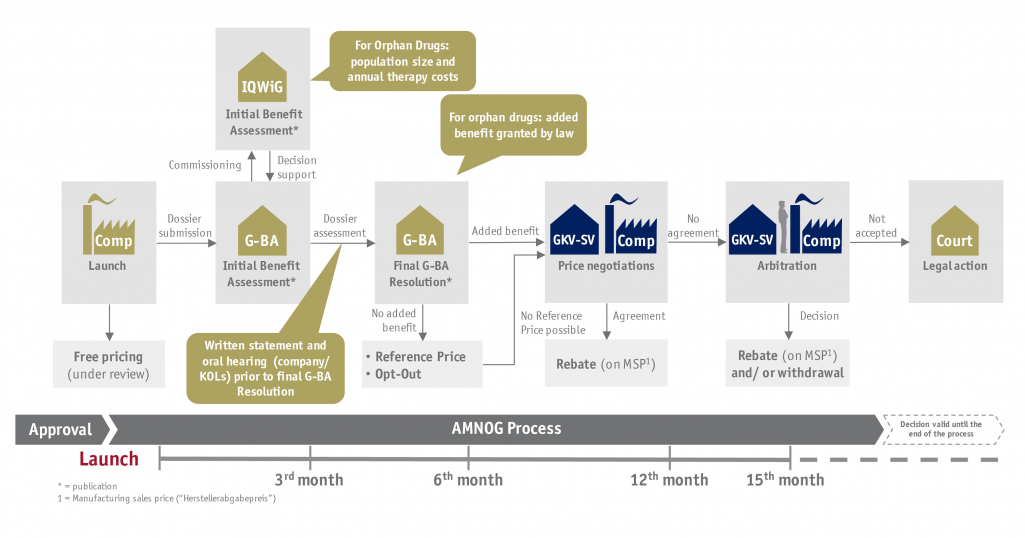
Reimbursement decisions for new innovative active agents are taken as part of a Health Technology Assessment (HTA) in most markets. In Germany, this is realized by an early benefit assessment according to AMNOG. In order to remain competitive, the pharmaceutical industry must be able to detect rapidly changing market conditions early and to adapt to such innovatively.
Companies within the pharmaceutical industry should have answers to the following questions:
- How can the value of the medical product be best positioned towards authorities, hospitals, or other institutions?
- Which pricing strategy can be implemented in European markets and remain financially viable?
- How can products contribute to competitive success after their patent protection expires?
- Which innovations will still lead to sustainable success in ten years?
- How might the entire business organization be required to adapt to the new challenges?
The solution
Strategy with long-term effect
A strategic approach at all stages of the HTA process is essential for ensuring a successful market entry and exploiting the full growth potential. An excellent strategic execution, its adaptation to changing conditions and the evaluation of markets are the basis for success.
Search for alternatives
Pharmaceutical companies must consider alternative reimbursement options, such as NUB, OPS, or DRG applications, or special contract models with payers and insurance companies.
Our approach
Experience that creates added value
We use our comprehensive care and industry expertise, our medical know-how, and our experience to provide strategic advice to clients from the pharmaceutical industry. With our excellent and ambitious team, we develop individual strategies and thus support sustainable success. We know the positions of payers well from numerous projects. We prepare for reimbursement processes in a targeted manner by applying stress tests to existing arguments early and support the client's position by convincing reasons.
-
Market Access
Development of practical and strategic expertise, such as for the preparation of internationally-focused market entry strategies (incl. AMNOG, NICE, SMC, AIFA, etc.)
-
Interim Management
Support as a strategic sparring partner up to taking over operative responsibility
-
Value Dossier
Development of argumentatively convincing value dossiers under consideration of value messages
-
Pricing negotiations
Simulation, strategic support and execution of pricing negotiations
-
Reimbursement strategies
Definition and processing of strategic fields of action for an optimal reimbursement
-
Pricing Workshops
Please find more information on our strategic pricing here.
-
Coaching
Support of our clients in the development of their business processes
Get in touch

Founder and Managing Director
Fax: +49 511 64 68 14 18