Feasibility analysis of a cost-benefit assessment for a gene therapy
Client: International research-based pharmaceutical company
Field of application: Oncology (orphan drug)
Challenge
The gene therapy of the pharmaceutical company had a very high price compared to other therapy options in the field of application. Due to the low price anchors provided by the comparable drugs, an additional basis for argumentation was sought to justify an adequate price in Germany. Conducting a cost-benefit analysis is one way of comparing and relating long-term economic and medical aspects of the treatment options in the area of application. Therefore, two main questions were addressed.
- Firstly, whether and, if so, to what extent a cost-benefit assessment is currently an acceptable instrument in the context of price negotiations or the AMNOG procedure in Germany.
- Secondly, whether, on the basis of the available or theoretically retrievable health care data, an advantageousness of the gene therapy over the other therapy options could be proven at all, i.e. whether there was cost-effectiveness.
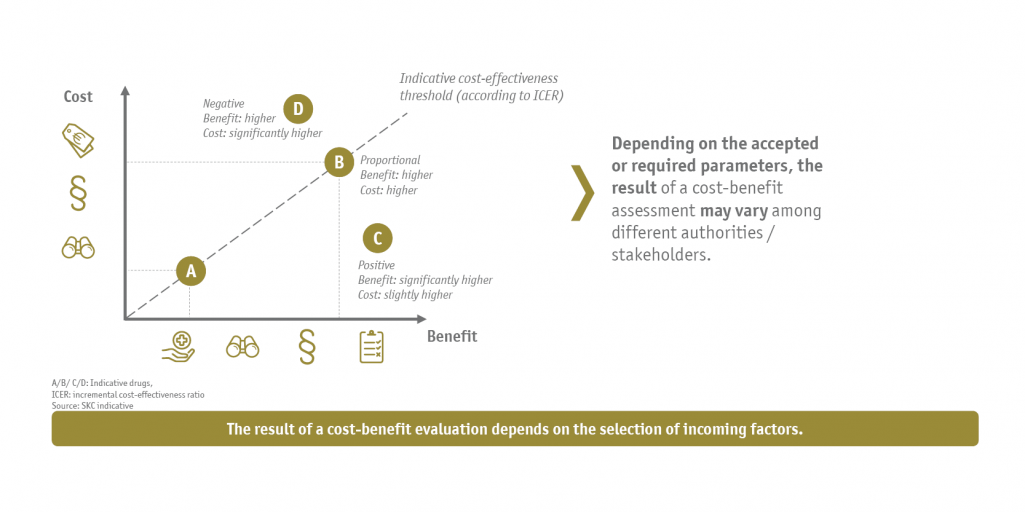
Solution and approach
To answer the questions, SKC first created an understanding of the general structure as well as the implementation of the different types of cost-benefit assessments, among other things by analyzing the methodology in an international context. In a second step, we identified which parameters and criteria are used by the relevant stakeholders of the German health care system (G-BA, IQWiG, arbitration board, GKV-SV and individual health insurance funds) for the acceptance of a cost-benefit assessment.
On the basis of these identified parameters or criteria and the available health care data, it was possible to derive a number-based estimate of the advantageousness or cost-effectiveness of the gene therapy compared to the comparable treatment options. By adapting individual parameters, it was possible to tailor and evaluate the cost-benefit assessment appropriately for the respective addressees. In addition, a sensitivity analysis was performed to investigate critical parameters and their singular influence on the economic targets.
Added value
It was shown that a mechanically clearly structured cost-benefit assessment can be significantly different depending on the context, in particular on the addressee of the assessment, the timing within as well as outside the AMNOG process, and overall with regard to the availability and use of data. The company gained specific insights into the short- to long-term cost-effectiveness of the therapy in different scenarios as well as a number-based foundation in the form of an Excel model that can be used as a decision support tool for cost-benefit assessments in upcoming price negotiations.