G-BA advice for an innovative gene therapy
Client: Emerging pharmaceutical company with a gene therapy as its first product after acquisition by a larger company.
Application: One-time intravenous gene therapy for the treatment of a very rare hereditary neuromuscular disease.
Challenge
Innovative gene therapies can offer patients with severe diseases an alternative treatment option. Unfortunately, they also carry risks for the patient because adverse events and especially long-term side effects and their triggers are unknown. However, gene therapies can mean significant improvements or even a cure for the patient after only one administration, which also means an enormous relief for doctors and the healthcare system.
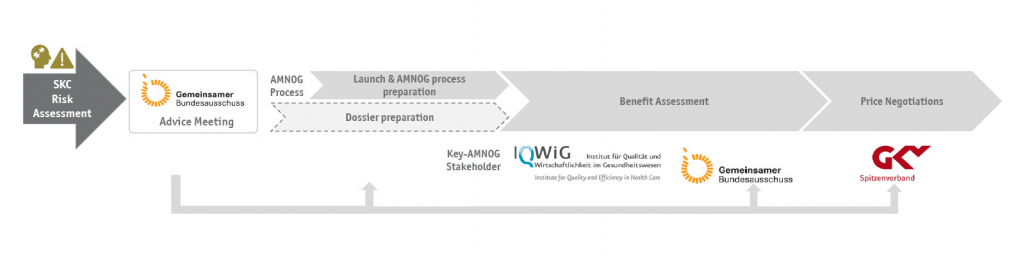
The pharmaceutical manufacturer (pU) bears all risks during the development of innovative gene therapies. Gene therapies with a one-time administration could reduce the costs of the GKV-Spitzenverband (GKV-SV) over a long period of time (possibly lifelong). However, for the pU, the costs of research and development must also be recouped and an appropriate return on investment must be generated. A good outcome can also be achieved through alternative reimbursement models like pay for performance. The basis for approaching a high reimbursement amount in a negotiation with the GKV-SV is a successful G-BA process.
Additional challenges for the pU due to the market entry in Germany are the high-quality requirements for gene therapies demanded by the G-BA as well as possible requirements for data collection accompanying the application (so called "anwendungsbegleitende Datenerhebung") or a registry. These must be fulfilled by the pU to ensure that the drug reaches the patient.
To achieve a successful market access in Germany, the client had commissioned SKC consulting to clarify crucial questions in a G-BA advice at an early stage in order to subsequently be able to address them up to market access.
- Is the study duration sufficiently long for the here present disease?
- Are the endpoints patient-relevant and sufficiently validated for the here present disease?
- Is the planned population size in the study (noting the prevalence) sufficient to achieve additional benefit in a direct comparison of treated patients to control patients?
- What is an appropriate comparator (relevant if the 50 million Euro threshold is exceeded)?
Solution and approach
In terms of identifying and valuating all challenges for the client regarding the AMNOG process, a comprehensive risk assessment is the solution. Following this, open questions can be clarified with a G-BA advice, so that specific requirements can be considered in the study planning and AMNOG process preparation. It is crucial to select the questions and formulations strategically so that the G-BA does not take the consultation as an opportunity to make further demands.
Another advantage of an early G-BA advice for this client was that the disease, the product and also the pU could be introduced to create a trusting relationship. The company was able to position itself to the G-BA as an open-minded and reliable company with an effective and innovative product for a disease with high unmet medical need.
Added value
SKC consulting conducted a systematic risk analysis by using a comprehensive grid to examine all important aspects of the AMNOG process. The task was successfully handled by SKC consulting with the help of its many years of experience and the large number of different AMNOG processes handled.
By looking at the product and the company from the outside, SKC consulting was able to identify and evaluate risks and opportunities, such as how the high-quality requirements for gene therapies can influence the success of the product on the German market or how to deal with the uncertainty of a possible requirement for a registry. Study-specific questions could be clarified in a G-BA advice. Also due to specific queries by SKC consulting, strategically important comments were reached by the G-BA and recorded in writing, so that the first foundation for successful market access had been laid.