Operational dossier creation with tight timeline (AMNOGk, § 14)
Client: Top 10 – global, research-focused pharmaceutical company
Application area: Oncology, multiple myeloma
Challenge
After a recent data cut showed favorable overall survival results in a previously assessed study, a request was made for a new benefit assessment based on new scientific evidence. Internal project timelines of the client required a rapid dossier preparation within 10 weeks after project start.
Solution and approach
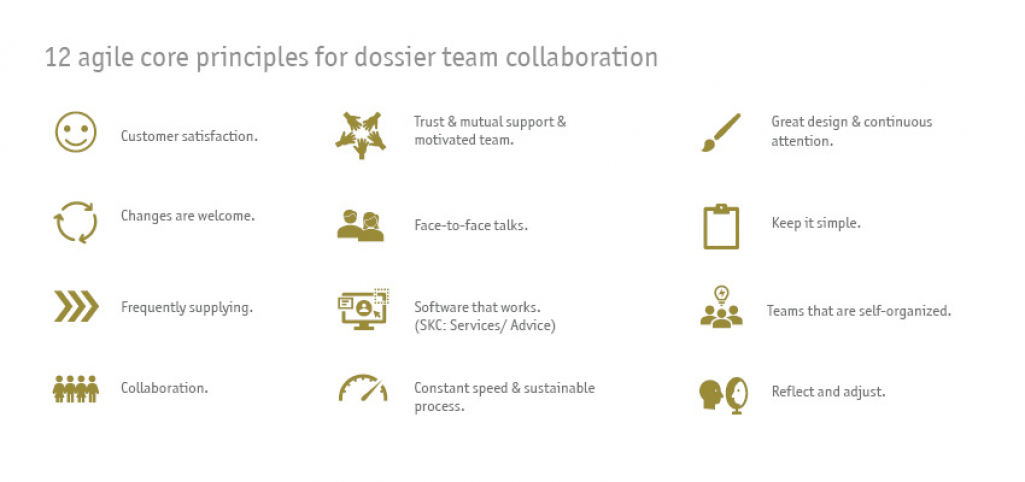
After reviewing the previous dossier, SKC consulting worked closely with the client to create an outline of the sections of the dossier that needed to be adjusted. In particular, required statistical post-hoc analyses and updates of the derivation of the patient population size were identified and communicated by SKC consulting at an early stage. Short lines of communication both with the client and within the SKC team, a clearly structured allocation of tasks, and accurate project overviews enabled timely acquisition of missing data and flexible response to changing priorities and timelines. Within 10 weeks, SKC consulting updated the entire dossier for a reassessment of the benefit. The objective of a timely submission of a complete and high-quality dossier for benefit assessment was successfully realized.
Added value
Applying agile working methods, it was possible to react flexibly to changing conditions and to submit a complete and high-quality dossier in close collaboration with the client despite a tight project schedule - with minimal effort and maximum satisfaction for the client.