Negotiation of the reimbursement amount in a reassessment after the deadline
Client: International pharmaceutical and healthcare company headquartered in Europe
Field of application: Ophthalmology
Challenge:
The pharmaceutical company engaged us to develop a negotiation strategy for a product that has been on the German market for several years and whose current reimbursement amount was set by the arbitration board. The decision on the benefit assessment had been limited by the G-BA in order to re-evaluate data collected in the context of a registry. Due to methodological deficiencies, the new data submitted for the re-evaluation were not considered for the assessment of the extent of added benefit. Subsequently, the existing added benefit was confirmed and the company was challenged to defend the reimbursement amount determined by the arbitration board several years ago without additional data recognized by the G-BA.
SKC was assigned to develop the negotiation strategy together with the company's market access team and to prepare and accompany the reimbursement amount negotiations.
Solution and approach
- Detailed analysis of the initial procedure and the reassessment (benefit assessment incl. G-BA resolution, justification, and oral hearing) as well as further precedents of reassessments after the deadline - using 12 years of AMNOG experience to anticipate the possible position of the GKV SV.
- Defense of the new data, which were not accepted for methodological reasons, but represent the best-possible evidence, and clarification of the therapeutic success, which is seen due to the use of the drug within the past years.
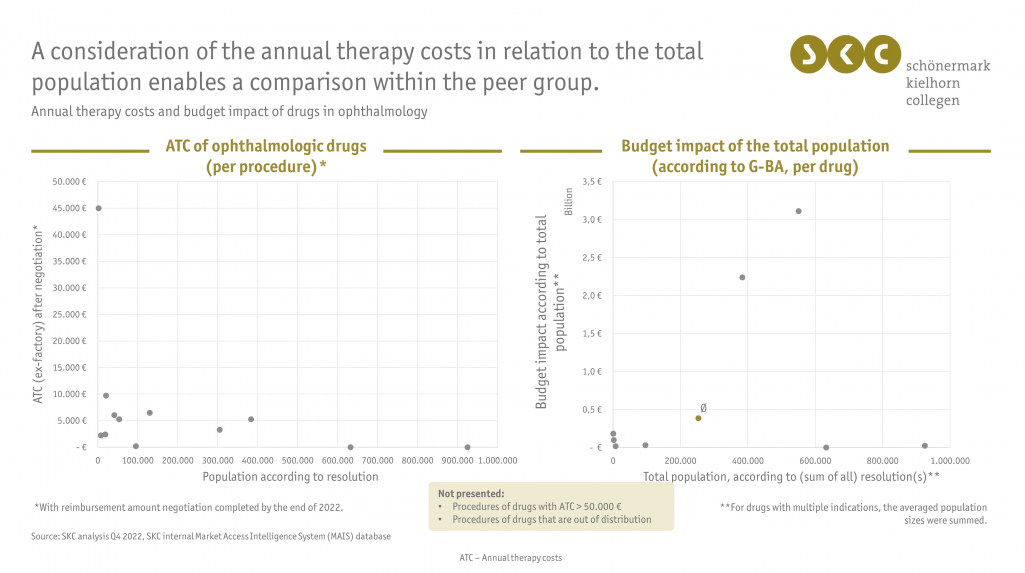
- Perform a peer group analysis: is the price set by the arbitration board in line with other comparable (currently) negotiated ophthalmologic drugs?
- Updating the European prices compared to the time of the first price negotiation and subsequent development of the strategic handling of changes.
- Based on this, develop a customized negotiation strategy adapted to the pain points seen in the first failed negotiation or arbitration board decision, which can be flexibly adjusted during the negotiation.
Added value
The longstanding use of the product and the positive treatment experiences by physicians and patients supported by the positive study results strengthened the value story of the product, which could be used as a starting position for the negotiations. By using a comprehensive analysis of the initial trial and further precedents as well as the presentation of a peer group analysis together with the relevant price factors according to the framework agreement – EU prices and monetarization of the added benefit – a stringent overall strategy could be developed and applied in the reimbursement amount negotiation.