Reimbursement amount negotiation without a price anchor - challenges for orphan drugs
Client: Biopharmaceutical company focused on developing and commercializing therapies for rare genetic diseases
Area of application: Metabolic disease
Challenge
For drugs used to treat rare diseases, presenting directly comparative data for the benefit assessment is a significant challenge. Due to small patient populations or ethical considerations (e.g. conducting placebo-controlled studies in children), the requirements of the German Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) for demonstrating an added benefit often cannot be met. Even when presenting the best-possible evidence, the level of evidence is frequently low, making it difficult for the G-BA to quantify the added benefit. Nevertheless, orphan drugs have a granted added benefit by law, which serves as the basis for subsequent reimbursement amount negotiations. Additional price anchors in such negotiations include comparable drugs and European reference prices, though the latter will be eliminated with the enactment of the Medical Research Act.
SKC was commissioned by one of its clients to jointly develop a strategy for reimbursement amount negotiations and to support the upcoming negotiations with the National Association of Statutory Health Insurance Funds (GKV-Spitzenverband, GKV-SV). The drug whose reimbursement amount was to be negotiated is used to treat a rare genetic disorder that severely impacts patients’ health from early childhood and for which no alternative therapy is available. Particularly challenging in such cases is the lack of a quantifiable appropriate comparator therapy and often no comparable drugs. Frequently, existing European reference prices are deemed not meaningful by the GKV-SV and will be excluded as price anchors by lawmakers soon. In addition to a low level of evidence in the benefit assessment and a resulting non-quantifiable added benefit, the challenge lay in developing an appropriate pricing strategy with almost no regular price anchors. The goal was to achieve the best possible outcome for the client by developing a value story and pricing strategy that is also comprehensible and acceptable to the GKV-SV.
Solution and approach
- Presentation of the burden of disease and unmet medical need in the specific indication, highlighting the therapeutic success of the medication, and development of a convincing value story that addresses the pain points of the GKV-SV.
- Comprehensive analysis of the benefit assessment process, including the G-BA resolution, justifications, and oral hearing, to extract the presented evidence and added benefit achieved by the medication to use this information in presenting the drug. Simultaneously, potential weaknesses that the GKV-SV might identify should be anticipated and addressed to develop appropriate counterarguments in advance.
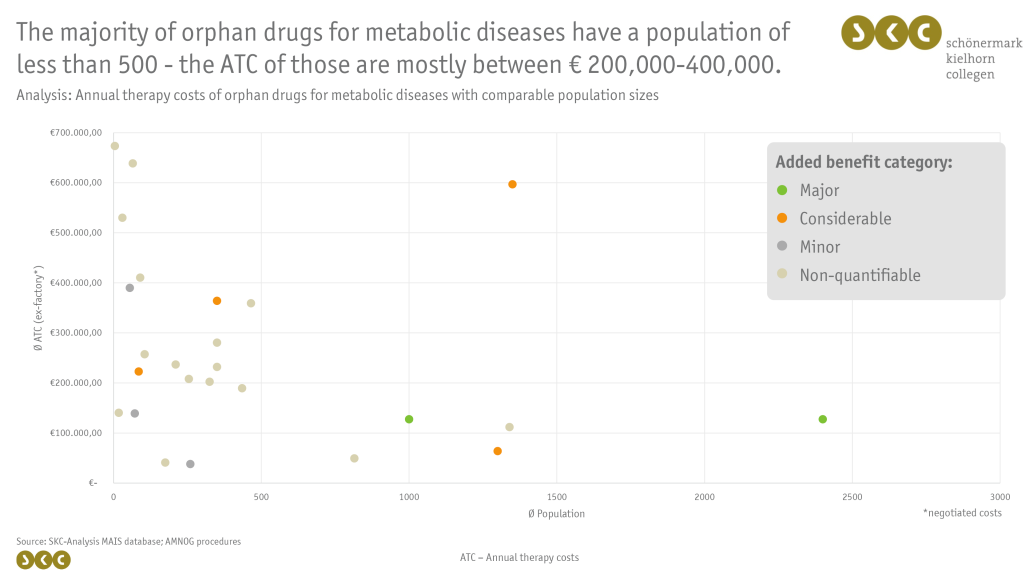
- Utilization of SKC’s proprietary Market Access Intelligence System (MAIS) database: Generation of suitable peer groups to identify analogous drugs (e.g., orphan drugs for other metabolic disorders with similar population sizes) and their costs, which can serve as price anchors by comparing therapeutic success and evidence. These peer groups can also be used to better estimate the willingness-to-pay of the statutory health insurance.
- Using the defined strengths of the medication, a tailored negotiation strategy is developed, adapted to the anticipated pain points of the GKV-SV, which can be flexibly adjusted during negotiations. This strategy should not only include the reimbursement amount but also other negotiation objects such as the price-volume regulation and contract duration.
Added value
The financial pressure on the statutory health insurance has been high for years. Therefore, it is increasingly important, especially in reimbursement amount negotiations for drugs without fixed price anchors, to achieve a willingness to negotiate with the GKV-SV’s negotiation team by clarifying the value of the therapy and contextualizing the therapy and its costs. This can be achieved through a stringent negotiation strategy, a clear presentation of therapeutic values, and classification within a comparable peer group.
Through the optimized utilization of the SKC-internal MAIS database, combined with years of AMNOG experience, particularly with orphan drugs, peer group analyses can be effectively performed and profitably applied. Simultaneously, intensive preparation of valid value arguments and responses to anticipated pain points of the opposing side helps to react to all eventualities within the price negotiations.