Discussion about mixed prices receives new breeding ground
Background: According to § 130b, para. 3 social code book V (SGB V), a new active substance that has not proven any added benefit may not be more expensive than the appropriate comparative therapy (ACT) specified by the G-BA. However, in the case of active substances with added benefits, the amount refunded is a kind of surcharge on the costs of the ACT. The necessity of a so-called mixed price occurs in case of a non-uniform subgroup evaluation, which is not intended according to §130b SGB V. This means that there are subgroups which have been granted with and without an added benefit. Experts and the arbitration board share the opinion that in such case mixed prices need to be set which cannot be calculated with a fixed algorithm, but instead must be negotiated individually.
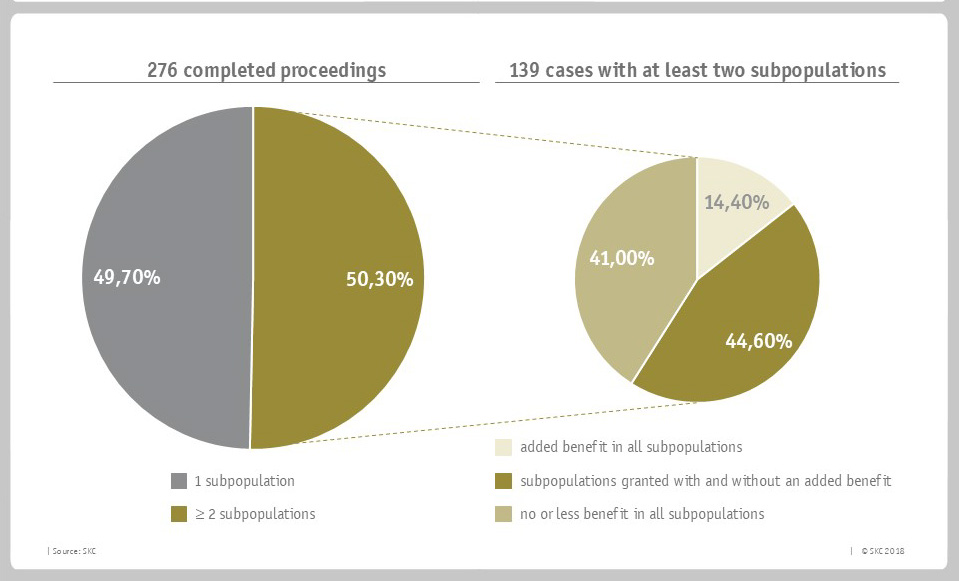
In an analysis of all proceedings completed till the end of 2017, SKC found out that in 50.3% of all cases, the G-BA had formed at least two subpopulations for one active substance. Only 14.4 % of these were considered to have an added benefit in all subpopulations, while 44.6 % show the previously described situation of a wide variety of additional benefit (subpopulations granted with and without an added benefit).
Since the verdict of the LSG Berlin on 28.06.2017 it is questionable whether mixed prices are legal. In the former proceeding, the National Association of Statutory Health Insurance Funds (GKV-SV) sued the arbitration board for pricing the active substance Albiglutid (Eperzan®) from the pharmaceutical company (pc) GlaxoSmithKline GmbH & Co. KG in the indication diabetes mellitus type II. As a result, one of five subpopulations has been hinted with a minor added benefit. The G-BA had estimated the number of patients in the subpopulation with an added benefit with about 30%, while this population had been estimated by the arbitration board with a weight of 80 %. In their judgement, the LSG explained that the calculation method has not been transparent and comprehensible and that mixed prices generally miss any legal basis. The same applies to the active substance Idealisib (Zydelig®) from the pc Gilead Sciences GmbH in the indication chronic lymphocytic leukemia. The arbitration board requested an appeal to the BSG, but the final judgement is still pending.
However, with these now published heterogeneous results by the authors Greiner and Witte, it is also clear that in case of mixed prices, an exclusive reference to the estimated prevalences by the G-BA is no longer sufficient, because they do not always reflect the reality of prescriptions.
SKC is the leading strategy consultancy for the healthcare sector in Germany and will keep you up to date on all the news about the AMNOG-process. We are happy to assist you with our expertise, for example in dossier writing in the context of the early benefit assessment or in the strategic support of price negotiations, where scenarios as described above will be discussed in detail and possible options will be developed. If you have any questions about mixed prices, please do not hesitate to get in touch with us.
Recent studies by the authors Greiner and Witte of the University of Bielefeld are giving the discussion about mixed prices in price negotiations for innovative drugs which have several subpopulations - with and without added benefit - new breeding ground. The previous mixed pricing calculations were either based on prevalence estimations (epidemiological model) announced by the Joint Federal Committee (G-BA) in its final decision, or market penetration assumptions. With the help of secondary data, the authors reveal that this prevalence estimation does not always correspond to the actual medical prescription in the individual subpopulation and, in some cases, significant bidirectional deviations can be detected.
Background: According to § 130b, para. 3 social code book V (SGB V), a new active substance that has not proven any added benefit may not be more expensive than the appropriate comparative therapy (ACT) specified by the G-BA. However, in the case of active substances with added benefits, the amount refunded is a kind of surcharge on the costs of the ACT. The necessity of a so-called mixed price occurs in case of a non-uniform subgroup evaluation, which is not intended according to §130b SGB V. This means that there are subgroups which have been granted with and without an added benefit. Experts and the arbitration board share the opinion that in such case mixed prices need to be set which cannot be calculated with a fixed algorithm, but instead must be negotiated individually.
In an analysis of all proceedings completed till the end of 2017, SKC found out that in 50.3% of all cases, the G-BA had formed at least two subpopulations for one active substance. Only 14.4 % of these were considered to have an added benefit in all subpopulations, while 44.6 % show the previously described situation of a wide variety of additional benefit (subpopulations granted with and without an added benefit).
Since the verdict of the LSG Berlin on 28.06.2017 it is questionable whether mixed prices are legal. In the former proceeding, the National Association of Statutory Health Insurance Funds (GKV-SV) sued the arbitration board for pricing the active substance Albiglutid (Eperzan®) from the pharmaceutical company (pc) GlaxoSmithKline GmbH & Co. KG in the indication diabetes mellitus type II. As a result, one of five subpopulations has been hinted with a minor added benefit. The G-BA had estimated the number of patients in the subpopulation with an added benefit with about 30%, while this population had been estimated by the arbitration board with a weight of 80 %. In their judgement, the LSG explained that the calculation method has not been transparent and comprehensible and that mixed prices generally miss any legal basis. The same applies to the active substance Idealisib (Zydelig®) from the pc Gilead Sciences GmbH in the indication chronic lymphocytic leukemia. The arbitration board requested an appeal to the BSG, but the final judgement is still pending.
However, with these now published heterogeneous results by the authors Greiner and Witte, it is also clear that in case of mixed prices, an exclusive reference to the estimated prevalences by the G-BA is no longer sufficient, because they do not always reflect the reality of prescriptions.
SKC is the leading strategy consultancy for the healthcare sector in Germany and will keep you up to date on all the news about the AMNOG-process. We are happy to assist you with our expertise, for example in dossier writing in the context of the early benefit assessment or in the strategic support of price negotiations, where scenarios as described above will be discussed in detail and possible options will be developed. If you have any questions about mixed prices, please do not hesitate to get in touch with us.
Sources:
https://www.aerztezeitung.de/politik_gesellschaft/arzneimittelpolitik/article/938925/urteil-mischpreisen-neue-arznei-fehlt-gesetzesgrundlage.html
https://www.aerztezeitung.de/politik_gesellschaft/arzneimittelpolitik/article/963305/erstattungsbeitraege-innovationenvfa-mischpreis-immer-kompromiss.html?wt_mc=socialmedia.Mail
http://www.lsg.berlin.brandenburg.de/media_fast/4417/Pressemitteilung_29062017.16427080.pdf
[/vc_column_text][/vc_column][/vc_row]
