„Not in a parallel world“
publication of the DiGA-V and the DiGA Guideline
Although colloquially referred to as an "app by prescription", these new DVG regulations not only include smartphone apps but they open the market to the statutorily insured for a wide variety of digital health care solutions. When the DiGAV ("digital-health application-regulation") came into force on the 20th of April 2020, the missing legal explanations, and definitions of the previously DVG were added. Thus, all prerequisites application procedures are now established and the legal foundation for the benefit assessment is set. According to the BfArM, applications for admission into the DiGA register will most likely be possible as soon as mid of May 2020. Considering the three-month deadline for fast-track-assessments the first digital health applications could be used and reimbursed as soon as of August 2020. The new regulations of the DVG grants the BfArM a fundamental role in the approval and evaluation process of digital health applications. Parallel with the entry into force of the DiGAV, the BfArM published a guideline to support manufacturers in preparing their applications describing the legislations and requirements for the proof of safety and efficacy in detail. However, the interpretation of the legal concept of „positive effects on care provision" mentioned in § 139e SGB V was expected with great anticipation.
The guideline was presented and discussed by several stakeholders at the virtual conference DiGa Summit. The authors pointed out the innovative nature of the new procedure, which resolves one of the substantial obstacles in the market access of digital health solutions, the cumbersome and time consuming path for digital health applications towards reimbursement, by introducing a so called "fast-track" with a significantly quicker assessment procedure. For this purpose, the assessment deadline for the BfArM is set to three months, starting with the submission of the complete application form by the manufacturer. The previously required time-consuming assessment processes, e.g. the assessment of new examination and treatment methods "NUB-assessment" according to §135 SGB V or the implementation of a "pilot regulation" according to § 137e SGB V are now substituted and replaced.
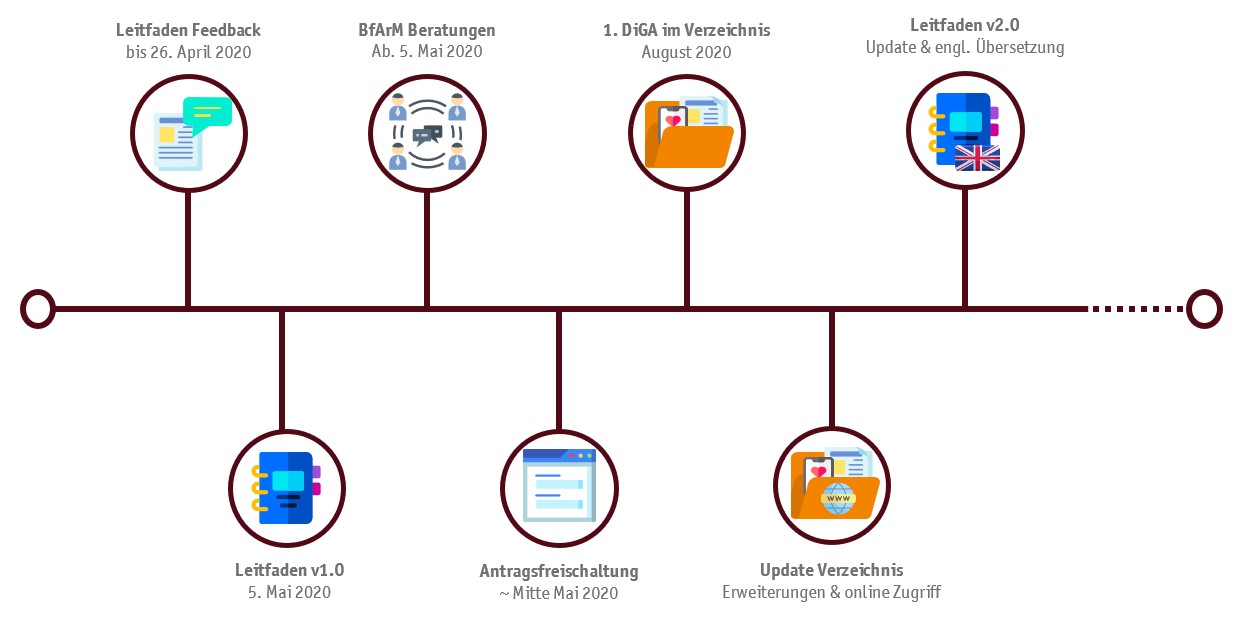
Source (BfArM 2020)
Considering the new assessment methodology of the "fast-track", elements of familiar reimbursement pathways could be identified. Furthermore, the new approach represents a combination of well-established assessment methods which have proven to be effective in the past. The AMNOG procedure can be seen as the foundation, although the evaluation authority of the Federal Joint Committee (G-BA)has been replaced by the BfArM. To accommodate the requirements for digital health applications and the often times-intensive efficacy proof, the requirements previously set by the G-BA for the AMNOG (especially in regards to proof of use by using patient relevant end-points) have been substantially mitigated and redefined as the "positive effects on care provision", causing a retrospective comparison to be sufficient. Additionally, facets of the pilot regulation have been implemented directly into the procedure to generate required evidence in the absence of sufficient clinical data.
During the Digital Summit, representatives of all partaking institutes assured their cooperation and agreement to the new fast track. Only Ms. Stoff-Ahnis from the National Association of Statutory Health Insurance Funds (GKV-SV) repeated her concern that a parallel world to current reimbursement regulations could be created. This point was taken up directly into the following BfArM statement and rejected with reference to the combination of the already existing procedures.
SKC will present a series of blogs explaining the new procedures and possibilities of the DVG in more detail and highlighting the regulatory specifications of digital health applications and strategic challenges for manufacturers. With the new DVG, the reimbursement possibilities for digital health applications will be fundamentally restructured. However, already well-established methods and working practices are taken over, in which SKC has successfully advised clients from the medical technology and pharmaceutical industries with extensive experience. Therefore, our team is looking forward to the new challenges and to the future support of medical device and pharmaceutical companies in the development of reimbursement strategies and benefit assessments of digital applications. The English version of the guidline is not available, but can be expected at the end of the year. In the meantime, please feel to contact us if you have specific questions regarding the application procedure or any other general requirements. We would be happy to support you.
German Sources:
- Leitfaden „Fast-Track"-Verfahren für digitale Gesundheitsanwendungen (DiGA)
- Verordnung über das Verfahren und die Anforderungen zur Prüfung der Erstattungsfähigkeit digitaler Gesundheitsanwendungen in der gesetzlichen Krankenversicherung (DiGA-V)
- BfArM-FAQ zum Fast Track für Digitale Gesundheitsanwendungen im DVG
- Health Innovation Hub – DiGA Summit – Zusammenfassung und Aufzeichnungen
