Medical prescription of digital health applications
unresolved issues & why the subsequent market strategy after the assessment procedure is crucial for manufacturers
At the end of August, the three-month deadline of the fast-track assessment procedure for the first wave of manufacturer applications for inclusion into the DiGA register is going to expire. According to a current report from the Federal Institute for Drugs and Medical Devices (BfArM), a total of 15 applications are currently being assessed simultaneously. The newly founded Central Association for Digital Health Care (SVDGV), in which the majority of DiGA manufacturers are organized, provides a current list of DiGAs submitted in a "provisional" DiGA register. The actual DiGA register is still under development by the BfArM. The register content should go beyond a mere listing and should enable healthcare providers to prescribe the most suitable DiGA for the current care situation. Apart from the precise medical indication and suitable patient populations, the proven positive effects of care provision, the duration of use and an explanation of the defined user roles for healthcare providers and patients should be specified. It is also intended to enable access to the assessed study data and, if applicable, the results from the pilot phase, as well as references for therapy procedures and implemented medical contents. A summary of the entire planned content can be found in the DiGA guidelines of the BfArM, which are now also available in English.
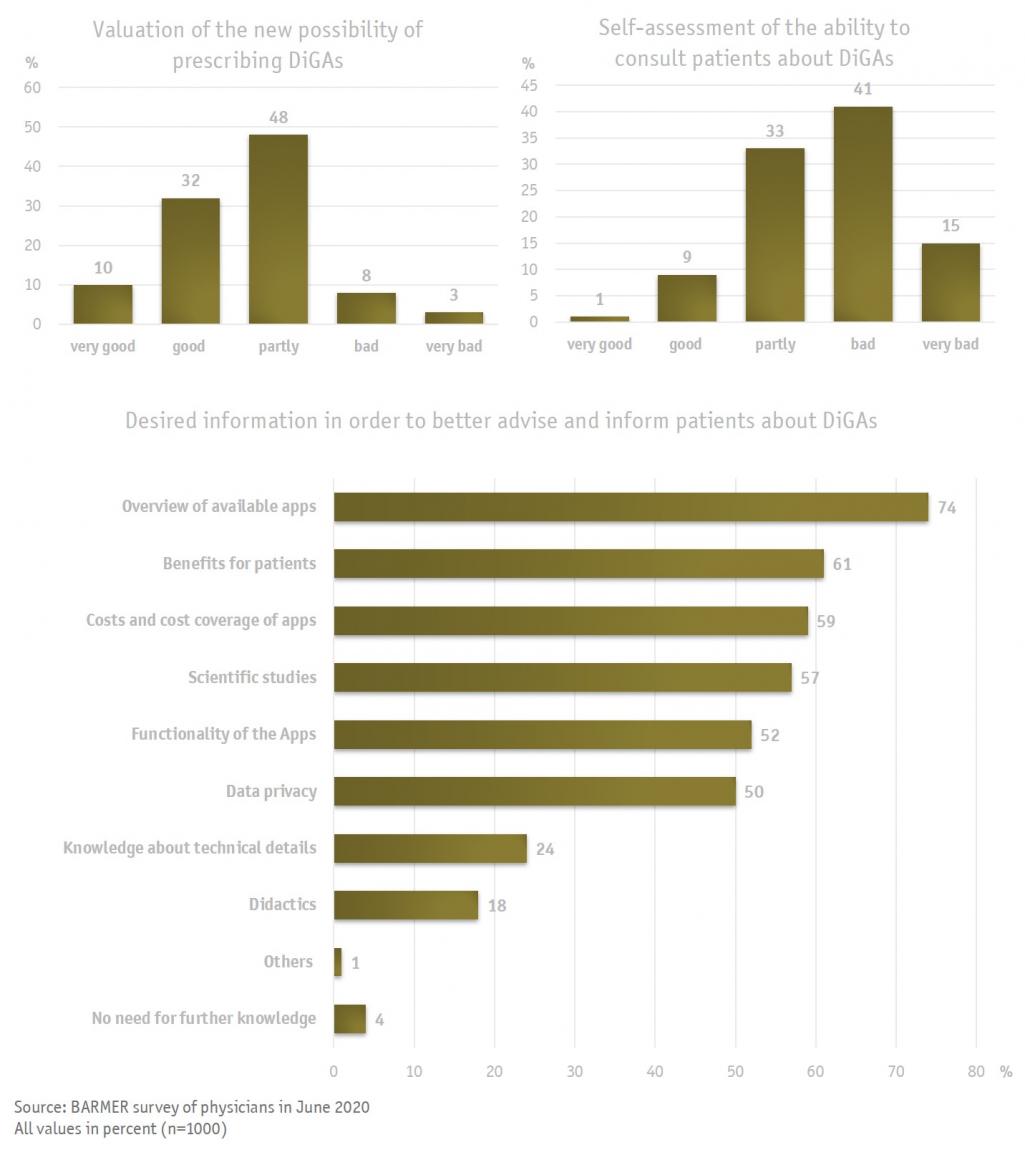
But how comprehensive will the displayed information be, and will they be sufficient for healthcare providers to justify a prescription? In a recent survey conducted by BARMER, 1000 physicians had a rather positive perception of the newly introduced DiGAs; only a small proportion evaluated the introduction negatively in general. However, it is also clear that some questions remain unresolved, and that essential information is missing. Only a small percentage of the physicians surveyed considered themselves well prepared for the upcoming consultations with patients, the majority (56 %) considered themselves poorly informed. This may contribute to the still missing DiGA register, which has not been published yet. A large part of the missing information mentioned will be covered by the DiGA register, although the completeness and clarity of the information cannot be assessed yet. The register must be finalized before the release of the first DiGA evaluation at the end of August. This gives the physicians limited time to familiarize themselves with the contents of the register.
Physicians will undoubtedly play a key role in the provision of DiGAs, even though patients can principally request DiGAs from their health insurance company: the submission of a confirmed medical indication is mandatory in any case. Therefore, it will be necessary for healthcare providers to take a closer look at function and operation principles of the relevant DiGAs in their specialty. For this reason, physicians demanded further information from the medical associations, the association of statutory health insurance physicians, the BfArM and health insurance companies, as well as from the manufacturers themselves, who should provide precise information about their DiGAs. It is still unclear who is going to ultimately instructs the patient in the usage of the DiGA and explains the functions in an understandable way. A part of the initial consulting effort will accrue to the physicians, who will also have to explain the basic features and the therapeutic goal of the DiGA. This provokes a major, and understandable, concern on behalf of the physicians, who fear considerable additional effort and insufficient remuneration. A precise and detailed instruction for the use of the DiGA cannot be provided by the physicians and psychotherapists. Although corresponding service codes are planned to be introduced in the Uniform Assessment Scale (EBM), they will not cover a thorough training.
Manufacturers are therefore confronted with two new challenges after successfully completing the Fast-Track process. On the one hand, they must introduce their DiGA into the prescription and invoicing cycle between patients, service providers and health insurance companies as well as position their DiGA strategically within the existing treatment pathways. In addition to the conviction of the involved stakeholders and in particular the healthcare providers of the therapeutic advantages of DiGA, the fast access to relevant and comprehensible information will be crucial. Manufacturers must provide specific information that explains the therapy contents and function of the DiGA in an uncomplicated way. Healthcare providers will only prescribe DiGAs if they are scientifically and medically convinced and have understood the underlying operation principle. A simple listing in the DiGA register will not be sufficient for the majority and will therefore not lead to a high market penetration. On the other hand, the manufacturers must close existing gaps in the instruction of their DiGAs. Thus, DiGAs themselves aim to improve therapy adherence, e.g. the use of medication, albeit they are also subject to a certain degree of required usage adherence. The initial explanation and instruction for the use of a DiGA as well as a competent contact person are closely linked to the adherence to long-term use. So far, mainly "early adopters" use DiGAs or ask their treating physicians about existing offers. This group is characterized by a high degree of self-initiative and motivation to familiarize themselves with digital technology and cannot be considered as a standard case for the entire patient population. A large part relies on more detailed instructions, which, in the event of failure, can quickly lead to a high drop-out rate, especially if a DiGA is prescribed without explanation. As a result, manufacturers should not remain satisfied with existing positive feedback from the health care market but should actively inform about their DiGAs and provide training material.
The way DiGAs are used in health care and whether they are used in the long term also affects the reimbursement. The legal basis already indicates that performance-based reimbursement models are desired and that the mere download of a DiGA will not be a sufficient parameter for reimbursement. Manufacturers must therefore consider strategies to convince users of their DiGA and at the same time encourage long-term use.
Sources in German:
- DiGA-Sprechstunde - Verordnung und Nutzung von DiGAs, 16. Juli 2020
- DiGA - Leitfaden Das Fast-Track-Verfahren für digitale Gesundheitsanwendungen nach § 139e SGB V
- Barmer-Umfrage zu Gesundheits-Apps – Ärzte stehen digitalen Helfern offen gegenüber
- Die Nutzerzahlen Digitaler Gesundheitsanwendungen (DiGA) stagnieren auf niedrigem Niveau
- Ärzteblatt: Digitale Gesundheitsanwendungen – Apps auf Rezept ab August