“With one′s back to the wall“ – How to convince the G-BA of an Added Benefit if the IQWiG is against you?
For further information on how to convince the G-BA of an added benefit if the IQWiG is against you, we would like to invite you to view our poster presentation at the Virtual ISPOR Europe 2020 conference which takes place from the 16th until the 19th of November
If a new drug is not granted an added benefit by the Federal Joint Committee (G-BA), its annual treatment costs (ATC) must not exceed those of the appropriate comparative therapy (= current standard of care). Depending on the situation, such a decision can therefore significantly jeopardize the business case for Germany and thus for all European countries. In the worst case, the pharmaceutical manufacturer cannot cover the costs and might even need to withdraw the drug.
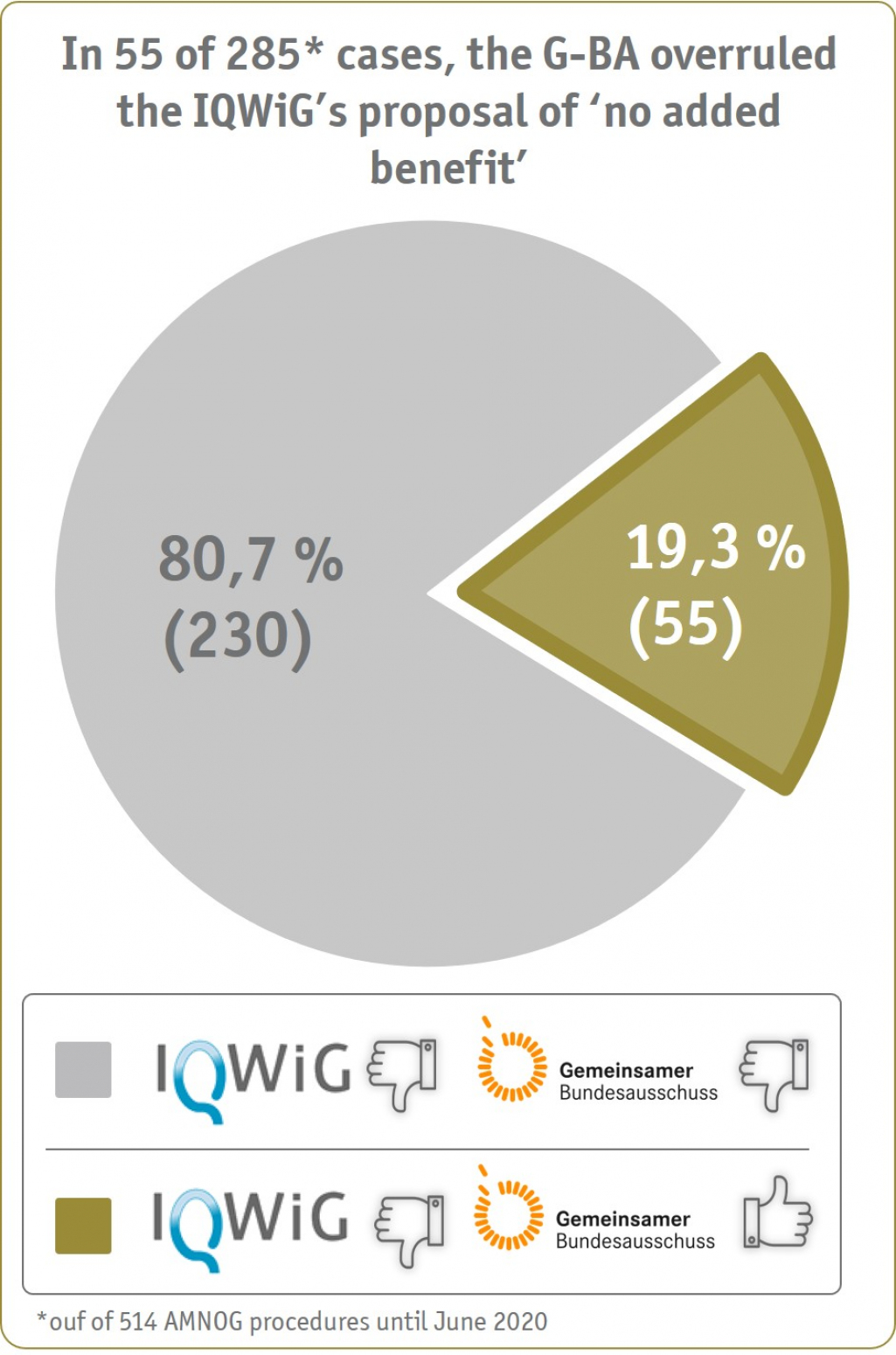
On the basis of the SKC database (combining the publicly available evaluations of IQWiG/G-BA with the results of the SHI price negotiations (LAUER-TAXE®)), the benefit assessment procedures were identified that were initially granted 'no added benefit' by IQWiG, but were subsequently overruled by the G-BA deciding for 'any added benefit' (until June 2020, see Figure 1).
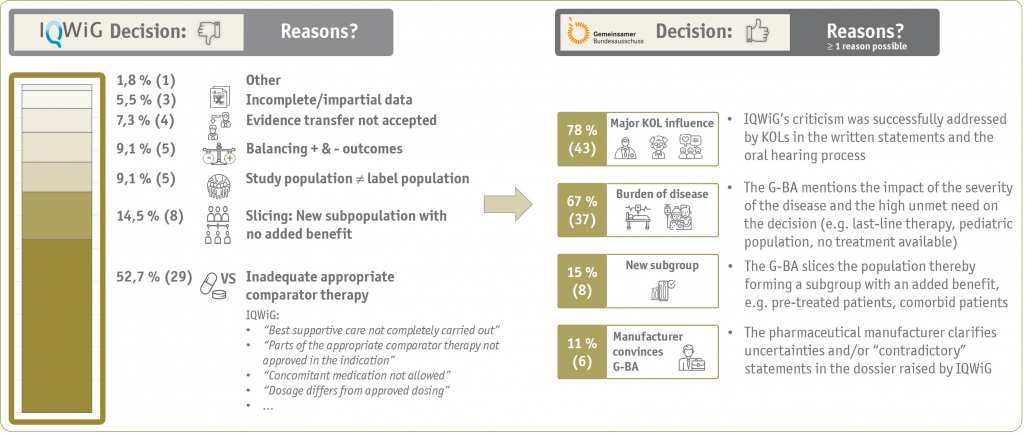
In more than 50 % of these cases, the IQWiG had initially questioned the correct representation of the appropriate comparative therapy (ACT) in the clinical evidence. However, due to the recent adaptations of the G-BA's rules of procedure now involving the relevant medicinal societies already in the early G-BA advice process to determine the ACT, the number of divergent decisions by IQWiG and the G-BA regarding the ACT is likely to decrease in the future.
Generally, in almost 80 % of all cases, a positive influence of KOLs on the G-BA was evident. Here, for example, the influence of KOL statements was mentioned in the G-BA's supporting reasons and/or resulted from the convincing and passionate arguments presented against IQWiG's criticism in the oral hearing (subjective view based on hearing protocols, see Figure 2).
The early involvement of KOLs and experts in the AMNOG process is therefore of crucial importance and can possibly "overcome" deficits in methodology and clinical evidence. However, this is obviously only possible to a certain extent - and requires the upfront identification and honest awareness of all possible weaknesses as part of the overall strategy. It is therefore crucial to anticipate the influence of these weaknesses on the decisions of the German HTA bodies right at the beginning of each Market Access Project.
To gain more information on this topic, please click here to view our poster presentation at this year's virtual ISPOR Europe.
About the author

Director Market Access
M.Sc. Life Science
Fax: +49 511 64 68 14 18