Successful Market Access for ATMPs
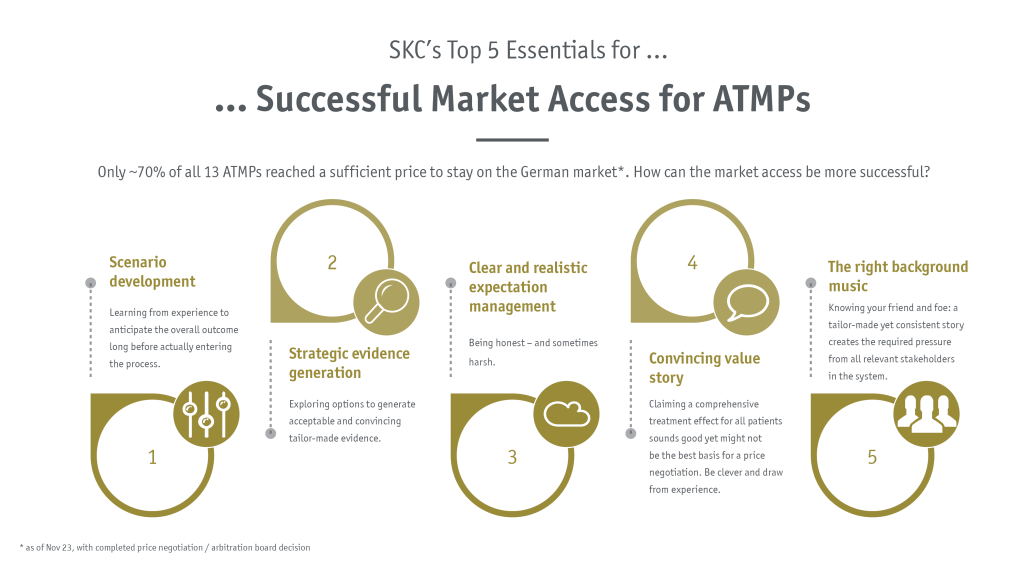
SKC’s top 5 essentials
Unrestricted approval and successful national negotiation
A successful market access can in general be defined as reaching as many patients as possible who can benefit from the drug and reaching the desired reimbursed and viable price level. The path towards unrestricted approval, a beneficial and valuable HTA (health technology assessment) rating and eventually a successful national negotiation is challenging – and in view of the EU HTA development it certainly became more uncertain for several manufacturers. From 12th of January 2025, all ATMPs approved by the EMA will undergo clinical assessment in the EU HTA followed by national negotiation and reimbursement decisions.
Overcoming specific obstacles
In general, the specific hurdles and challenges for ATMPs could be clustered and categorized in different topics: manufacturing, evidence generation, health economics, and regulatory and organizational aspects. Especially when it comes to HTA and pricing and reimbursement for gene therapies, there are in general two crucial aspects to consider.
- Firstly, the gene therapies often come with high promises up to a “one-shot” curative therapy which could save the continuous costs of the current standard of care, i.e., promise “cost-effectiveness” – in addition to the substantial value for patients burden of disease and quality of life.
- Secondly, the evidence level in general or at least at the time of approval is comparably low compared to other medicinal products. Especially due to the lack high-quality comparative data and the lack of long-term data, HTA bodies and payers in particular emphasize the high degree of uncertainty when it comes to the sustainability of the therapeutic effect. Exploring the advantage and individual applicability of different opportunities such as outcome based contracting options, alternative pricing models, a potential routine practice data collection and others is of utmost importance when planning the road ahead.
In the coming years, the number of gene therapy treatment options will multiply. CAR T-cell therapies remain with ~50% proportion the most common technology used in the pipeline of genetically modified cell therapies (preclinical through to pre-registration). In general, oncology and rare diseases remain the top areas of gene therapy development in both the overall pipeline (preclinical to pre-registration) and in the clinic (Phase I to pre-registration). The number of precedents is growing, and the system is learning & developing – both of which needs to be considered to successively challenge and optimize one’s individual strategic approach.
As a summary and in conclusion, comprehensive experience and expertise leading to the right, upfront-defined, and iteratively optimized market access strategy is an essential factor for success. SKC continuously monitors, anticipates and analyzes the outcomes of ATMPs and innovative drugs and includes all findings directly in the strategic consulting with clients.
Sources: SKC expertise and MAIS database
About the author

Director Market Access
M.Sc. Life Science
Fax: +49 511 64 68 14 18