Impact of dynamic changes of the definition of the appropriate comparative therapy (ACT) on market access performance of new active substances in Germany
Schikowski M., Gibson L., Mrosowsky T., Schönermark M.P., (2018):
Poster Presentation
Abstract
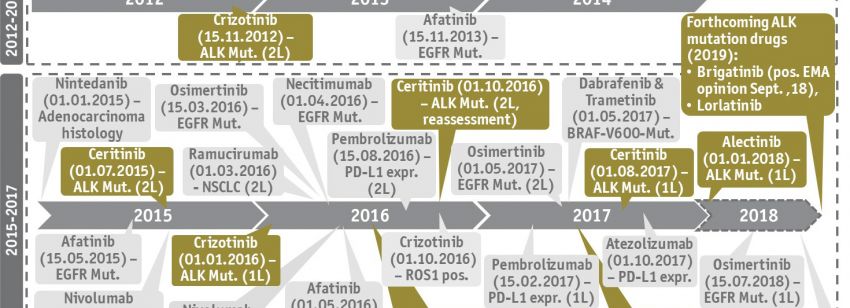
Since 2011, the Federal Joint Committee (G-BA) has assessed the added benefit of new drugs launched in Germany. The outcome of this assessment is the basis for reimbursement and price negotiations with the statutory health insurance. For orphan medicines, an added benefit is granted by law. For non-orphan drugs, the G-BA determines an appropriate comparative therapy (ACT) according to criteria defined in the G-BA Code of Procedure. New active substances regularly appear on the market, especially for indications with a somewhat' crowded' market. Hence, the ACT may change dynamically, even during already ongoing benefit assessment procedures.
Download
Here you can download our poster presentations free of charge.
Impact of dynamic changes of the definition of the appropriate comparative therapy (ACT) on market access performance of new active substances in Germany
PDF 187 kB Download