EU HTA: European Health Technology Assessment (JCA)
The challenge
Addressing the challenges of the EU HTA
A central feature of the EU regulation is the joint clinical assessment (JCA) of new medicines at European level. However, the decision on the additional benefit and the amount of reimbursement remains the responsibility of the respective member states at national level.
The German HTA authorities - the Federal Joint Committee ( Gemeinsamer Bundesausschuss, G-BA) and the Institute for Quality and Efficiency in Health Care ( Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG) - have had a significant influence on the implementation of the EU HTA Regulation for years through their involvement in various committees and planning groups as part of the work of the EUnetHTA 21 preparatory consortium: Both methodological and structural requirements in the implementation and design of the JCA process are substantially orientated towards the established German benefit assessment procedure.
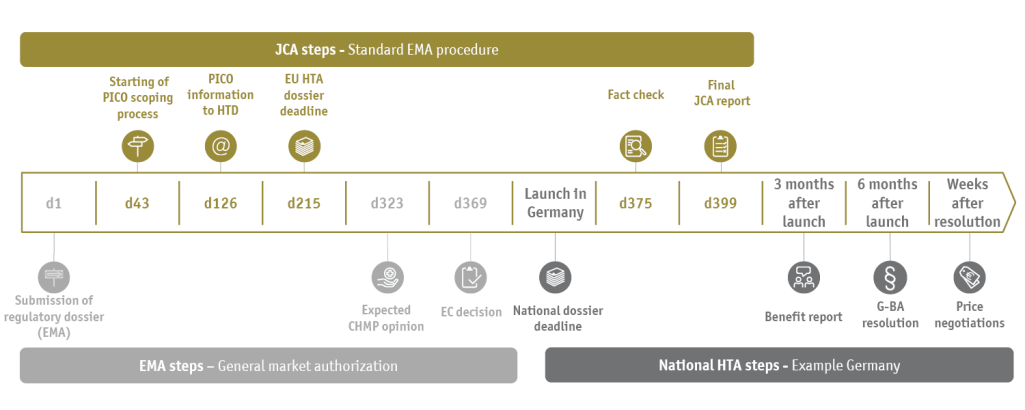
The JCA is a new process for the evaluation of clinical evidence at European level; it is mutually linked with the EMA authorization timetable (see figure).
In order to minimize risk and increase planning certainty, a number of key questions arise for pharmaceutical companies:
- How is the JCA process structured in terms of timing and organization? What interfaces are there?
- How do I need to prepare the process strategically and operationally? Which functions and expertise are required?
- How does the PICO scoping process work? Which scenarios are realistic and what do they mean for my overall success?
- Which methodological requirements apply? How can I best position myself if I cannot - or do not want to - fulfil them?
- How exactly is the European clinical evaluation carried out? How can I prepare the national procedures optimally?
- How can I efficiently coordinate the procedures at European and national level? How can I reduce the supposed complexity and general multifunctionality?
The solution
Comprehensive understanding through active participation in shaping the process
We at SKC have accompanied the development of the EU HTA process and the JCA from the very beginning by actively participating in stakeholder bodies such as the EUnetHTA 21 Stakeholder Meetings.
- As one of the most active opinion leaders, we contributed to the revision of the deliverables on the methodological and procedural design of the EU HTA process.
- We are an active member of the EUCOPE network and constantly liaise with the pharmaceutical industry to jointly develop solutions to procedural hurdles.
Leveraging an international network for European excellence
SKC has various reliable strategic and operational partnerships in Europe and worldwide.
- We work with national market access agencies in order to incorporate further local HTA and pricing expertise into the overall European strategy as required.
- In addition, we utilize a clinical network spanning all European member states in order to validate our hypotheses through clinical experience, e.g. on relevant PICOs. Through the clinical experience of our founder and managing director Matthias Schönermark, MD, this network is characterized by collegiality and offers significant added value.
- We also work with other functional experts as required, such as research analysts in the area of market and payer research or biostatisticians in the area of evidence strategy.
Experts for German market access - the blueprint for the JCA
Since the beginning of the AMNOG in Germany, we have successfully supported our clients strategically and operationally in the German benefit assessment and price negotiation process. Due to the central influence of the G-BA and IQWiG as well as the generally high quality standards and requirements, the German procedure has now become the blueprint for EU HTA. With our extensive experience in the German system and long-standing interactions based on mutual trust with the German authorities G BA and IQWiG, we are the perfect partner to successfully guide our clients through the European HTA process. Especially at the beginning of the implementation of the new EU HTA framework, it is important to draw on our extensive experience in the German AMNOG process in order to anticipate adjustments and implications and reduce uncertainties.
Agile working methods and active handling of innovations and uncertainties
In today's world, VUCA - volatility, uncertainty, complexity, ambiguity - is a key challenge in strategy development and implementation. A further European level now reinforces this. SKC utilizes the agile way of working in order to respond flexibly to unpredictable obstacles and changing circumstances. We are demonstrably certain that the agile way of working offers a significant efficiency advantage thanks to the high level of responsiveness and constant flow of information in the area of market access.
Our approach
As Market Access Special Forces, we support our clients in mastering EU HTA.
-
"Fit for EU HTA Mastery Workshop" - is your product portfolio fit for EU HTA?
In our Fit for EU HTA mastery workshop, we jointly analyze and discuss the general requirements and success factors of the new process and, based on this, collectively evaluate the specific strategic implications, for example for your portfolio and corporate structure. The workshop also includes an active self-assessment and a specific evaluation for the respective functions in the company. This approach has proven its worth in creating the necessary awareness as well as initiating sustainable tactical measures as part of the overall European orientation.
-
PICO scoping - reducing uncertainty
PICO schemes (P=Population, I=Intervention, C=Comparator, O=Outcome) define the scientific questions of clinical evaluation that need to be answered with the help of statistical analyses of study data in the JCA dossier. There can be fundamental differences between the member states in this respect. Depending on the indication to be assessed, there may be substantial differences in the standard of care within the EU, which means that the pharmaceutical company must provide several and possibly numerous PICOs with its study data. However, the requested PICO schemes are only made known to the pharmaceutical company 100 days before the JCA dossier is submitted (as of Feb 2024).
Due to the short timeframe for preparing the European dossier, a key success factor is defining valid hypotheses for the final PICOs requested and strategically evaluating these for their respective implications, for example for national price negotiations. In a gap analysis, the evidence package is formally and strategically analyzed with regard to the expected requirements and appropriate options for closing the gaps are derived. In this way, effort and benefit can be optimally balanced, for example with regard to the generation of further evidence.
-
Developing and implementing an anticipated EU HTA strategy
Once potential risks, opportunities and fields of action have been identified for specific products and clients, the next step is to address these fields of action in the form of developing and implementing an EU HTA strategy. This strategy incorporates the national price negotiations of the individual member states right from the start. We always start with the goal in mind: how can we successfully achieve the desired level of reimbursement while making the medicine available to as many patients as possible.
Together with our clients, we develop a roadmap in a strategy workshop for the implementation of concrete steps within the identified fields of action to improve the chances of success before and during EU HTA. We provide both strategic support in the creation of scenarios and operational support in the actual implementation of tactical measures. We accompany you through the EU HTA process as an approachable and reliable partner at eye level.
-
Creating a strategically complete and efficient JCA dossier
The European dossier requires the collation of large volumes of data within tight timeframes. This poses not only methodological but also technical challenges that need to be solved. SKC has developed a modular and AI-supported process ("JCA100") to meet the special requirements of creating the JCA dossier in 100 days. This innovative approach is based on the statistical calculation of PICO datasets, their orchestration and semi-automated textualization in accordance with the qualitative requirements of the JCA.
With its experience from numerous AMNOG dossier projects, SKC is the ideal partner for the implementation of the European dossier. Based on our extensive experience in dealing with the methodological requirements of the German authorities, which are high by international standards, we create an evidence presentation that meets the requirement criteria in conjunction with a value story tailored to the product.
-
Delta dossier - using the national delta smartly
The JCA dossier contains all necessary information for a European clinical assessment. At national level, additional or specific requirements often apply, for example with regard to the costs of the comparator therapies or the cost-effectiveness of the new therapy. These aspects will also be provided in a national dossier. As the interface between the European and national pricing strategy and as a decisive opportunity to anchor the national value story, the "delta" dossier is of considerable tactical importance. We support our clients both in providing advice and in preparing the necessary documents
-
Harmonizing European assessment and national negotiations optimally
To achieve the optimal strategic alignment for national benefit assessment and price negotiation, the strategy needs to be thought beyond the JCA. The joint clinical assessment based on multiple PICO schemes can create risks and opportunities that the pharmaceutical company should strategically consider for successful national HTA procedures. We analyze the implications of the JCA report for national assessment procedures and subsequent price negotiations for optimal preparation. Having provided strategic or operational support or managed more than 80 price negotiations in Germany, we know that early strategy development is crucial for the basis of national price negotiations and thus for successful market access.
Get in touch

Founder and Managing Director
Fax: +49 511 64 68 14 18