Successful Market Access in Oncology
SKCs Top 5 Essentials
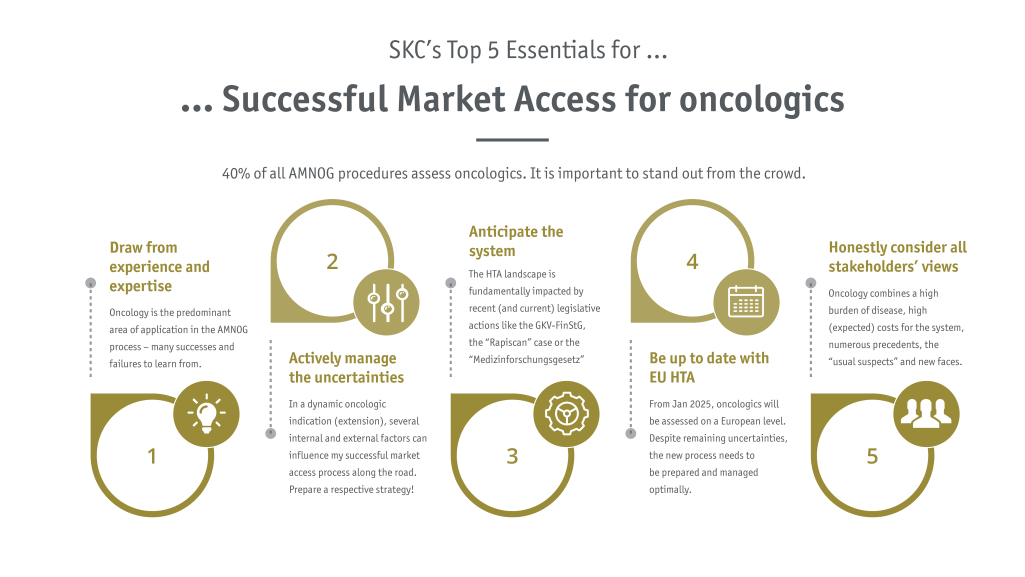
The term oncologics refers to all drugs that are used to treat cancer. However, the spectrum here is extremely broad and diverse, both in terms of the disease and the medication. The individual indications include the rather mild forms with many treatment options, the highly refractive therapy situations with no further options and no realistic chance of survival as well as the rare oncological indications. At the same time, the spectrum of drug types ranges from more generalist and comparatively simple approaches such as platinum-based therapies, to more effective immunotherapies and innovative gene therapies such as CAR-T cell therapy.
With regard to market access in Germany via the AMNOG procedure, it is therefore crucial to draw the right conclusions based on the findings from the appropriate procedures, or to reject them on the basis of reasonable and acceptable arguments. However, looking at the past alone is not enough. For one thing, oncology is a very dynamic field with many innovative developments. The changed (competitive) situation at the foreseen time of the HTA process and price negotiations must be anticipated at an early stage and taken into account for the development of a successful market access strategy. In addition to the therapeutic development in the respective oncological indication, the overall development of the system must be closely monitored and evaluated for potential implications, such as adaptations of the HTA assessment guidelines or adjustments in the framework of the price negotiations. Unforeseen surprises are not wanted.
In addition, oncology drugs are under particular scrutiny, especially in the current and upcoming times: in the difficult financial situation of the German health insurance system, oncology drugs are often highlighted as particularly cost-driving, for example through combinations or, supposedly, step innovations. This rather subjective yet clear view, as well as the position of all other stakeholders, must be taken into account when setting prices and developing a negotiation strategy.
In order to be jointly successful in the end, i.e. to achieve the desired reimbursement price and to be able to help as many patients as possible with the drug, SKC continuously monitors and analyses the AMNOG procedures and price negotiations for all oncology drugs and incorporates all findings directly into the strategic advice provided to clients.
Sources:
About the author

Director Market Access
M.Sc. Life Science
Fax: +49 511 64 68 14 18