EU HTA benefit assessment: more tightly timed than suspected
Update on the Joint Clinical Assessment (JCA) timeframe
First draft of the concrete timeline
Due to the large number of different PICO (Patient, Intervention, Comparator, Outcomes) schemes for which data will have to be submitted by the manufacturer, the European dossier will be significantly larger and more time-consuming than dossiers at the national level. EUnetHTA21, the consortium of national HTA bodies assigned with preparing the EU HTA process until September of this year, has reacted to the uncertainties regarding the timeframe of the European assessment by setting up a hands-on group on timelines for Joint Clinical Assessments (JCAs). This group has now published the first draft of the concrete timeline for the European assessment process.
Two EU HTA timeline scenarios
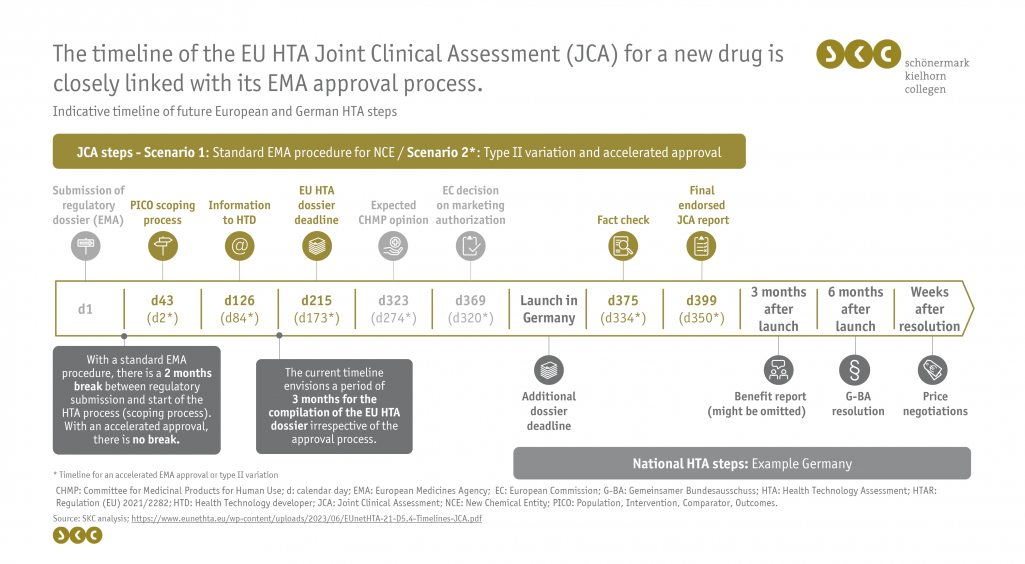
The fact that the European HTA process of a new drug depends primarily on the timeline of its European Medicines Agency (EMA) approval is already laid out in the EU HTA Regulation. The legal text defines that the European submission dossier must be submitted by the manufacturer at least 45 days before the envisaged date of the Committee for Medicinal Products for Human Use (CHMP) opinion. The draft of the hands-on group now distinguishes between two EU HTA timeline scenarios depending on the respective EMA approval process:
- a joint clinical assessment (JCA) adjacent to a standard EMA procedure for new chemical entities (NCEs) and
- a JCA with an accelerated approval procedure or for type II variations (e.g., indication extensions).
The timeline of the assessment process is shown in the following figure for both scenarios.
The European clinical assessment starts with the submission of the regulatory dossier to the EMA (Day 1). The coordination group then initiates the scoping process, which does not start until 42 days later in the case of a standard procedure but is initiated directly in the case of an accelerated approval procedure or a type II variation. The result of the scoping process is communicated to the pharmaceutical companies after about 2.5 months (day 126 or day 84), which marks the beginning of the tight timeline for the preparation of the European benefit assessment dossier. It is striking that with 3 months, the timeframe from the disclosure of the PICO schemes to the submission of the EU HTA dossier is equally short in both scenarios (day 215 or day 173). If the hands-on group had used the latest possible deadline of 45 days before the CHMP Opinion for the submission of the dossier, as allowed by the legal text, the time to prepare the dossier would amount to about 5 months.
With the granted approval, the launch of the drug in Germany is possible, which subsequently starts the national benefit assessment. Within 30 days after approval, the final JCA report is submitted to the manufacturer to check for factual errors and is then published in its final endorsed form approximately 11 to 13 months after the initial submission of the approval documents.
The uncertainty remains
Although it is of great value for pharmaceutical companies to obtain initial insights into the timeline of the EU HTA process, the published timeframe only represents a first draft. It is still being revised and will also be up for discussion at a meeting of EUnetHTA21 with health technology developers on July 13th, 2023. Furthermore, possible innovations in the EMA approval processes due to the recently proposed adaptation of the EU pharma legislation are also not yet taken into account.
We are pleased to keep you informed about all relevant developments regarding the EU HTA process. If you are interested in discussing the implications of the published EU HTA timeline for the market access of your product or looking for ways to prepare the EU HTA dossier under the given time critical conditions, please feel free to contact us. We are the market access special forces.
Sources:
About the author

Senior Medical Writer
M.Sc. Drug Research and Development
Fax: +49 511 64 68 14 18