Impact of dynamic changes of the ACT on market access performance of new active substances in Germany
About the author

Prof. Matthias P. Schönermark, M.D., Ph.D.
Founder and Managing Director
Founder and Managing Director
Fon: +49 511 64 68 14 – 0
Fax: +49 511 64 68 14 18
Fax: +49 511 64 68 14 18
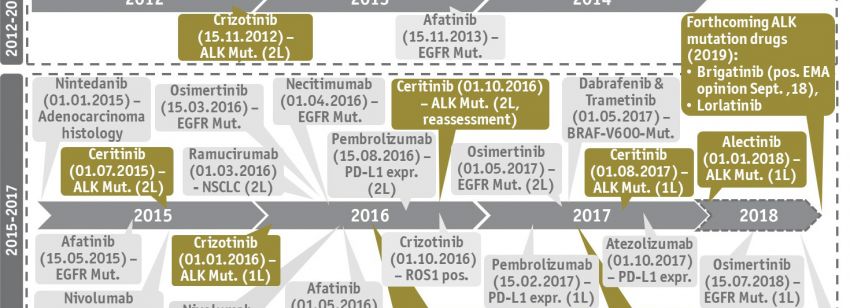
In 276 proceedings concluded from 2011 until 2017 we analyzed 105 proceedings on active substances in oncology, 39 proceedings in diabetology, and 21 proceedings in neurology. Non-small cell lung cancer or melanoma were the indications with the highest volatility for which the G-BA had granted an additional benefit compared to the respective ACT in several cases. However, these HTA outcomes directly influenced some cases of ongoing HTA due to a sudden change of the ACT in a specific indication. In other disease areas, e.g. diabetology, most new medicines were not granted with an added benefit resulting in no change of ACT for several years. Similarly, ACTs in disease areas with a very small number of HTA proceedings, such as schizophrenia or epilepsy, were stable over many years.
At the ISPOR Europe 2018 a poster from Michèl Schikowski, Lydia Gibson, Ph.D., Thora Mrosowsky and Prof. Matthias P. Schönermark M.D., Ph.D. regarding the impact of dynamic changes of the ACT on market access performance in Germany was presented in Barcelona. We at SKC are interested in the long-term strategic development of the pharmaceutical market and continuously monitor the ongoing HTA proceedings in Germany which may give rise to great advantage for market access strategies. We would be pleased to support you with our expertise for your product launch in Germany.
BY Prof. Matthias P. Schönermark, M.D., Ph.D., managing director and Thora Mrosowsky, M. Sc. Health Economics