New ways into the trial phase for medical technology
amendment of § 137e SGV and the implications thereof
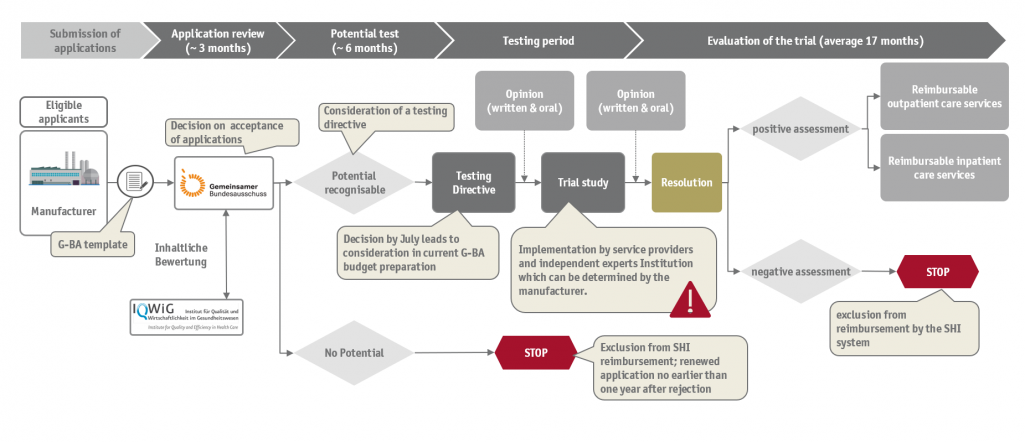
In retrospect, the motivation for the introduction of the trial rule was that medical technology innovation could be more quickly incorporated into regular reimbursement and costly evaluation procedures such as a NUB procedure according to §135 SGB V could be avoided. However, the very first years after the implementation in 2012 showed that the process is associated with some bureaucratic and costly hurdles. It was not until 2017 that the first trial phase was launched, more than 5 years after the actual entry into force. The manufacturer must also prove that a corresponding influence on the independent scientific institution can be excluded. The Federal Joint Committee can also still appoint an independent institution on their own.
In addition to minor procedural innovations, medical device manufacturers are now entitled to commission their own independent scientific institution to provide scientific support and evaluation of the test at their own expense. According to the new procedural regulations, however, separately assigned study concepts must be submitted and approved by the Federal Joint Committee. The subsequent procedures will remain unchanged. For manufacturers, however, the main advantage lies in their own initiative to commission study concepts. This could speed up the process from the potential identification onwards, as study concepts could already be developed in parallel allocation procedures.
Corresponding changes have now been implemented in the seventh section of the second chapter of the VerfO, bringing the regulations into force immediately. The regulation can be used retroactively for all procedures that started with the trial until May 11, 2019. Whether the change will lead to a significant acceleration is still unclear, but the amendment will at least partially reduce the bureaucratic hurdles in the procedure, which could increase the attractiveness for medical device manufacturers. SKC gives comprehensive advice along the testing of new examination and treatment methods and supports clients in consulting requests for potential assessment and application preparation.