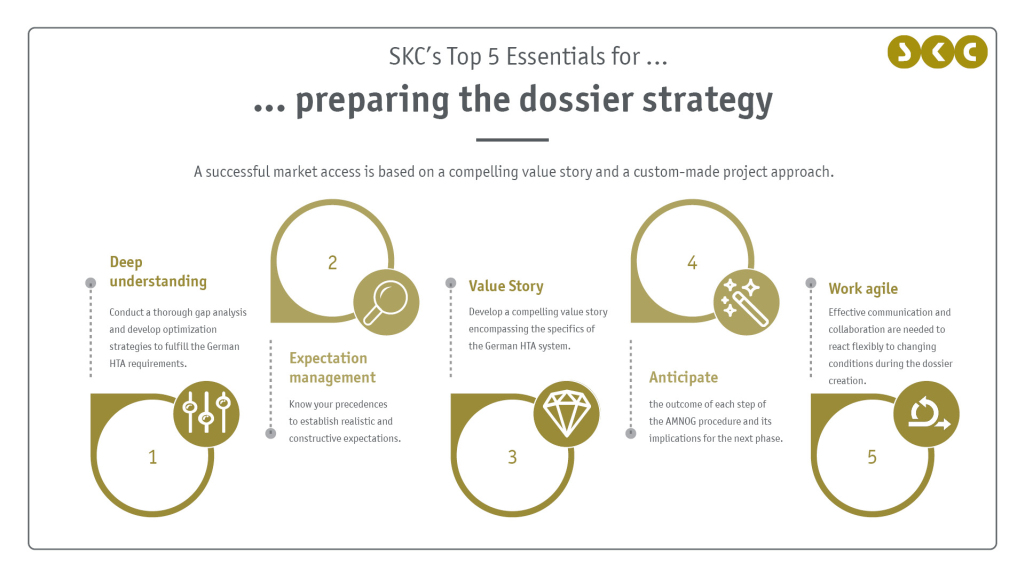
Preparing the dossier strategy
SKC’s top 5 essentials
The value dossier covers information on the patient population size, burden of disease, available (comparative) therapies, therapy costs, study results and more; and often consists of thousands of pages. The outcome of the dossier-based benefit assessment by IQWiG and the German Federal Joint Committee (G-BA) lays the ground for the subsequent price negotiation with the statutory health insurances.
Hence, the dossier strategy needs to be well prepared. Experience in all steps of the AMNOG procedure and knowledge of the German HTA requirements are crucial to develop a comprehensive and compelling dossier strategy. Especially for German market access novices and in non-standard cases such as orphan procedures, the commission of an experienced strategic sparring partner can tip the scales and pave the way for a successful market access in Germany.
At SKC, we think from the end of the AMNOG procedure and anticipate potential challenges and solutions from the beginning. With the help of our very own database MAIS, which encompasses details of all previous AMNOG procedures and their outcomes, we have access to a unique set of information and can provide you with valuable precedences. Our interdisciplinary and experienced team is not only prepared to support you strategy- and content-wise, but our agile operating principles are also well suited to promote effective communication and collaboration within your company as well as with stakeholders such as the G-BA, KOLs and patient advocacies. Thereby, we ensure the generation of a compelling value story, that fits the specifics of the German HTA system, and a custom-made project approach for a successful market access of your product.