EUnetHTA 21 deliverables
Timelines for facilitating a future EU HTA system
The EUnetHTA 21 consortium consists of the following national HTA bodies, led by the ZIN (The Netherlands):
- AEMPS (Spain)
- AIFA (Italy)
- AIHTA (Austria)
- G-BA (Germany)
- HAS (France)
- INFARMED (Portugal)
- IQWIG (Germany)
- KCE (Belgium)
- NCPE (Ireland)
- NIPN (Hungary)
- NOMA (Norway)
- TLV (Sweden)
- ZIN (The Netherlands)
Since it was established in September 2021, EUnetHTA 21 follows up on the gains and insights of the previous projects and focuses on facilitating a future EU HTA system. This is urgently needed as the European regulation on HTA (EU HTA regulation) came into force on January 11th 2022 and tertiary legal acts as well as guidance documents are currently not available.
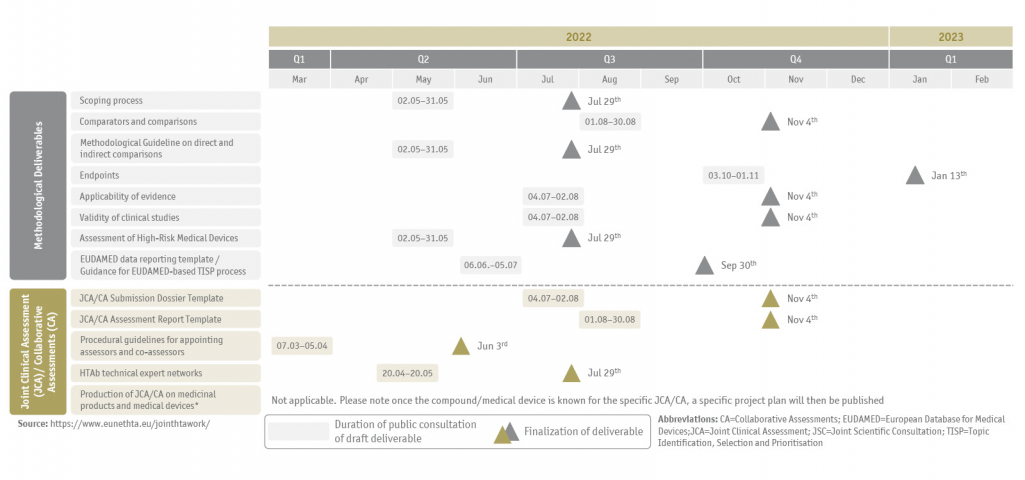
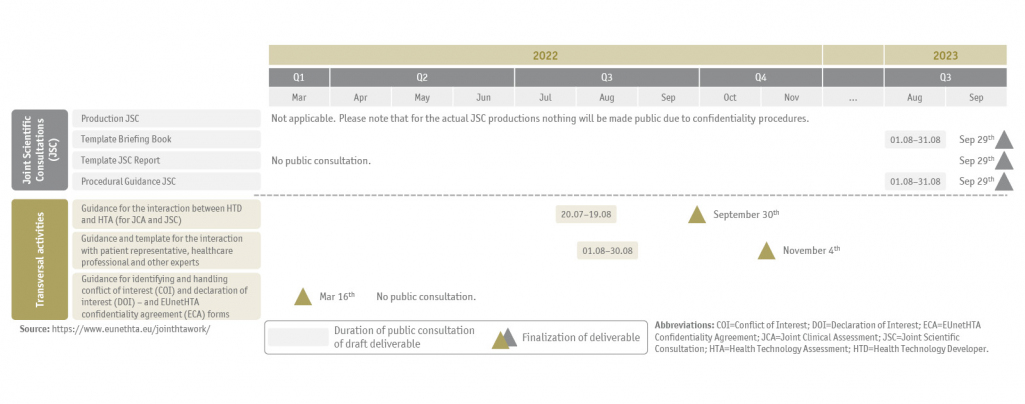
All deliverables, the current timeline for public consultation and finalization of draft documents are summarized below (Figure 1 and Figure 2) and regularly updated on the EUnetHTA webpage.
EUnetHTA project plan timelines
SKC closely follows the development of guidance documents and actively participates in the public consultations. Thus, on May 30th 2022 we have submitted our comments on the draft sub-deliverables D4.2 Scoping process and D4.3.2 Methodological guideline on direct and indirect comparisons. If you are interested in our position on the draft sub-deliverables, we would be pleased to hear from you and will be happy to provide you with our written statement on request.
We encourage all stakeholders to also get involved to facilitate the smoothest possible transition from national to EU HTA and ensure a clear and effective guidance for the European assessment of health technologies.
Sources: