EUnetHTA 21’s second open call for applications for Joint Scientific Consultations
On the way to implementing the European regulation on HTA
Having this timeline in mind, clinical phase II and/or III studies for medicinal products for which European approval is anticipated as of 2025, should already be planned in accordance with the new requirements that the EU-HTA entails. Given that the publication of many guidance documents is still pending, participation in an advice meeting is of particular importance.
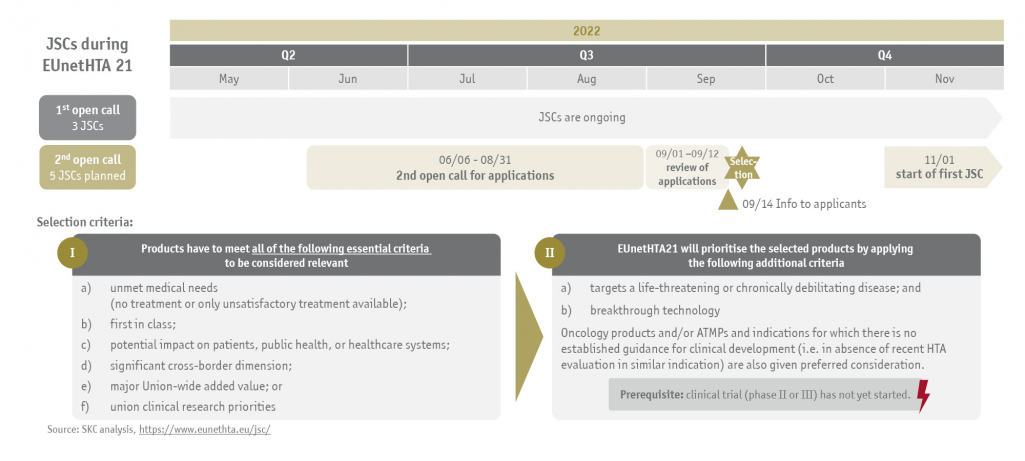
While the regulation is not implemented, the EUnetHTA 21 consortium is assigned to perform six to eight Joint Scientific Consultations (JSC) until September 2023. Three medicinal products have already been selected for an advice meeting and their JSCs are currently ongoing. The second open call for applications for the remaining maximum of 5 JSCs starts on June 6th, 2022, and ends on August 31st, 2022.
Mandatory selection criteria for medicinal products to be considered for a JSC are as follows:
- unmet medical needs (no treatment or only unsatisfactory treatment available)
- first in class
- potential impact on patients, public health, or healthcare systems
- significant cross-border dimension
- major Union-wide added value or
- union clinical research priorities
EUnetHTA 21 will then prioritize applications based on the following additional criteria:
- targets a life-threatening or chronically debilitating disease and
- breakthrough technology
Oncology products and/or ATMPs and indications for which there is no established guidance for clinical development (i.e. in absence of recent HTA evaluation in similar indication) are also given preferred consideration.
The following illustration shows the process for Joint Scientific Consultations during EUnetHTA 21:
In case your company is currently planning a phase II and/or III study for a product which meets these criteria, or you are unsure whether your product qualifies, SKC would be excited to hear from you. Moreover, in case you have already booked an EMA Scientific Advice, we would be pleased to discuss options for switching the advice into a Parallel EMA/EUnetHTA 21 JSC. We at SKC look forward to supporting our clients in the application, preparation, conduct and debrief of a JSC in our well-known nature as strategic advisor and sparring partner!
Sources:
EU-HTA regulation
EUnetHTA 21