Chronological orchestration of EU HTA and national benefit assessment of medicinal products
European Health Technology Assessment (EU HTA)
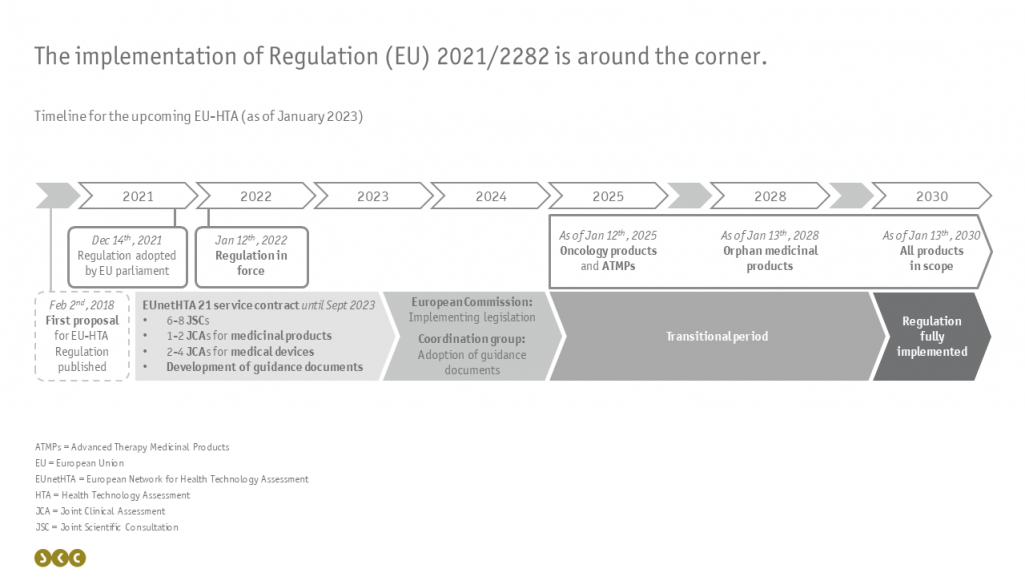
The implementation of these joint clinical assessments will take place gradually from January 12th, 2025, and is planned over a longer period of time: Beginning with oncology products and Advanced Therapy Medicinal Products (ATMPs) in 2025 to the full range of affected medicinal products in 2030 (see figure).
In the meantime, the EUnetHTA network - a consortium of various national HTA authorities - is commissioned with conducting trial runs for consultations and JCAs and producing guidance documents complementary to the regulation. Subsequently, the resulting documents and findings will be translated into implementing legislation by the European Commission and adopted as further guidance documents by the Coordination Group.
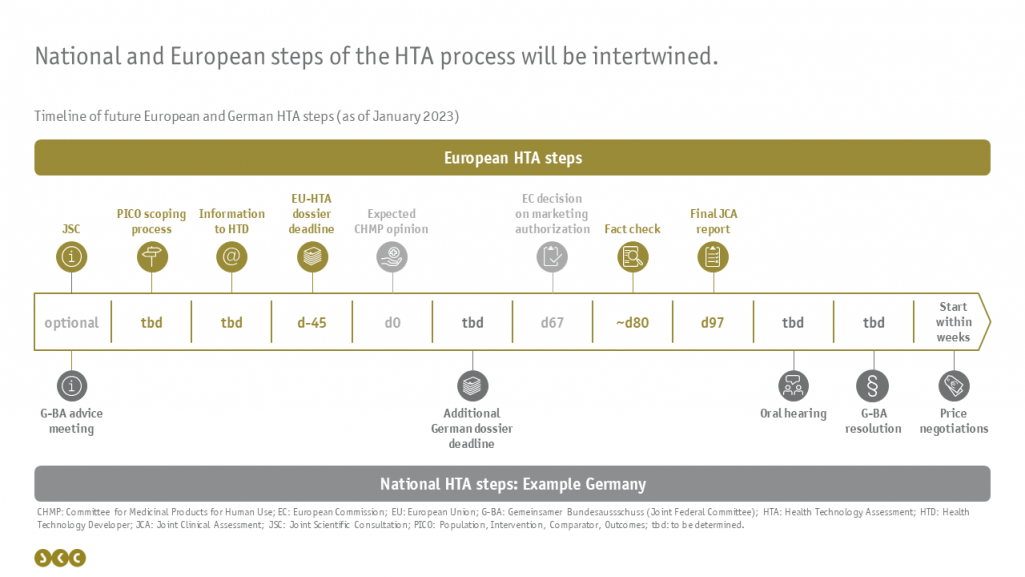
The timing anchor of the JCAs at European level will be the expected date of the CHMP opinion: Already 45 days prior, the dossier must be submitted for joint clinical assessment at EU level. For this purpose, the health technology developer (HTD) will be informed after a consultation of the individual EU member states (scoping process) which PICO schemes must be fulfilled in the dossier, i.e. for which patient populations, interventions, comparators and endpoints results must be submitted. However, this notification is likely to occur at very short notice (possibly only 55 days before the dossier submission deadline), leaving the HTD little time to prepare the dossier. Accordingly, it is worthwhile to make use of a voluntary consultation in advance: either at the European level as a JSC (Joint Scientific Consultation) or separately at the national level in the EU member states.
30 days after approval of the medicinal product, the European JCA report is published, after the HTD has had the opportunity to comment on it once with regard to factual errors. Comments on the content or subsequent submission of documents as known from the written statement of the German AMNOG process will not be possible.
The decision on the additional benefit of the medicinal product as well as the price negotiations remain the responsibility of the individual member states. Since it is already becoming apparent in Germany that not all the information needed for the decision of the German Joint Federal Committee (G-BA) will be included in the European dossier, it is almost certain that an additional, supplementary dossier will have to be prepared for Germany. This possibility exists for all member states. Which countries will make use of this and when these supplementary modules will have to be submitted has not yet been determined. In general, it is still unclear how the European and national steps of the procedure will interlock. In addition to the mandatory steps of the HTA procedure, the G BA has already announced that it will continue to hold an oral hearing.
We are pleased to keep you informed about new information and will be happy to support and advise our clients on the strategic market access of their medicinal product or medical devices under both the current national and the future applicable European legislation.
Sources: