SKC analysis after BSG ruling
46 AMNOG benefit assessment procedures could be unlawful
Until a corresponding change in the law is made, this ruling may have implications for all future and also past benefit assessment procedures in which therapeutic soloists were assessed, i.e., drugs with new active ingredients for which only therapies without approval can be determined as appropriate comparative therapy (ACT) in the given indication.
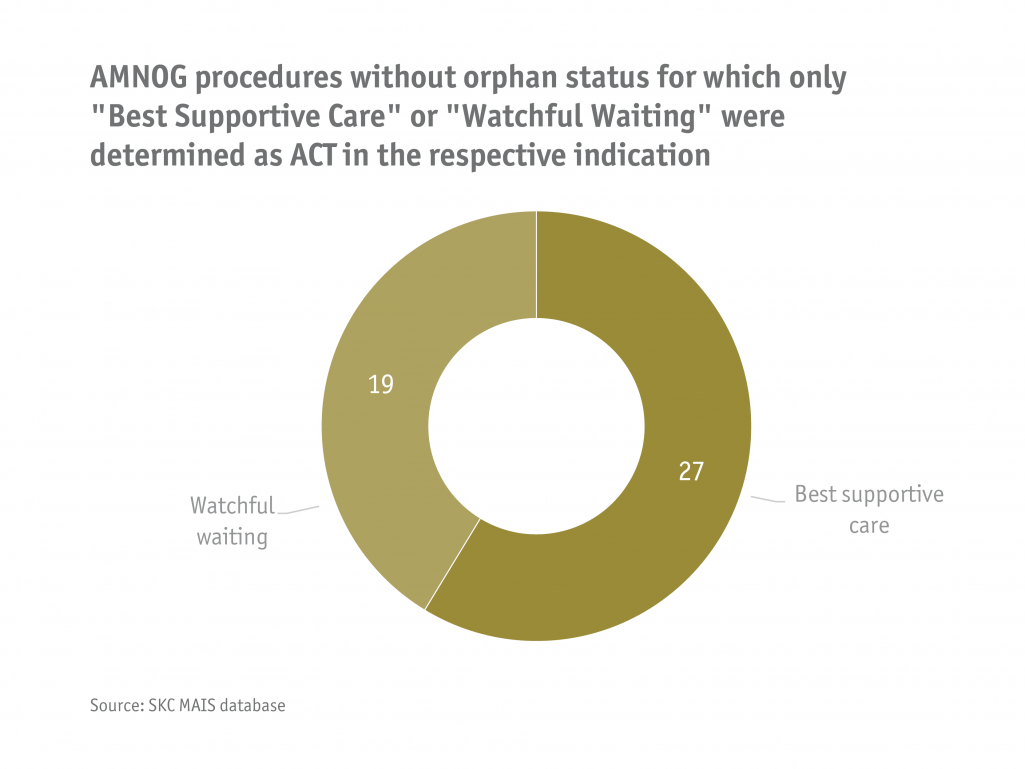
In an analysis using SKC's propietary MAIS database, all AMNOG procedures without orphan status were identified for which only "Best Supportive Care" or "Watchful Waiting" were determined as ACT in the respective indication. The result was 46 benefit assessment procedures (27 procedures with ACT "Best Supportive Care" and 19 procedures with ACT " Watchful Waiting"), which were conducted in the period August 2012 - February 2023. Based on the ruling of the BSG, it is highly probable that the benefit assessment of the active substances concerned in the respective area of application was unlawful due to their soloist status. These procedures may now have to be reversed.
In the case of Erivedge®, Lonsurf®, Mulpleo®, Nerlynx®, Orkambi®, Palforzia®, Sialanar®, Slenyto®, Stivarga® and Zinplava®, there are also no further benefit assessments in other indications with an ACT that is lawful according to the BSG. Thus, the price regulation based on the price negotiations with the GKV-SV, as in the case of Rapiscan®, would have to be considered completely ineffective and instead the price freely set by the manufacturer would have to be reimbursed.
SKC will continue to monitor the impact of the BSG's ruling in the Rapiscan® case on the benefit assessment procedure in Germany. At the moment, the legal extent of this far-reaching decision does not yet appear to be assessable.
Sources:
- MAIS-Datenbank (kombiniert AMNOG Verfahren und Lauer-Taxe)
- SKC blog: Reasoning of the BSG ruling underlines the special position of legal therapeutic soloists - G-BA calls for rapid legislative amendment
- SKC blog: German Federal Social Court: added benefit assessment for therapeutic soloists unlawful - The case of Rapiscan®
About the author

Director Market Access
M.Sc. Psychology
Fax: +49 511 64 68 14 18