One year price structure model
What does this mean for the reimbursement amount after expiry of patent and document protection?
Exactly one year ago, on 1 May 2022, a new section was added to the framework agreement between the German National Association of Statutory Health Insurance Funds (GKV-Spitzenverband, GKV-SV) and the associations of pharmaceutical companies in order to implement the legal amendment in § 130b section 8a and 9 of the SGB V from 1 April 2020. The amendment to the law specifies that when patent and document protection expires, the maximum permissible ex-factory price for a medicinal product is the last valid reimbursement amount. The framework agreement now defines the regulations necessary for the negotiation of a price structure model (PSM) between the originator and the GKV-SV. The PSM then enables manufacturers who want to market a medicinal product with the same active ingredient to determine the maximum reimbursement price. On the occasion of the first anniversary of the amendment of the framework agreement according to § 130b paragraph 9 SGB V, we at SKC have analysed the essential contents as well as the resulting strategic implications for the manufacturers of original products.
It is now just over twelve years since the introduction of the AMNOG. Therefore, in the coming years, the patent and document protection for more and more AMNOG products will expire and the pharmaceutical companies will have to negotiate a PSM with the GKV-SV. However, the amendment of the framework agreement came too late; the property rights for 19 AMNOG medicinal products had already expired beforehand. For these active ingredients, generic manufacturers had the option of setting a higher reimbursement amount than that of the original product, but this was only the case for one active ingredient when it entered the market (cabazitaxel), for which the price level was then quickly adjusted downwards again. In the other cases, the prices of the generic products, even before the introduction of the PSM, were generally below the reimbursement amount of the original product.
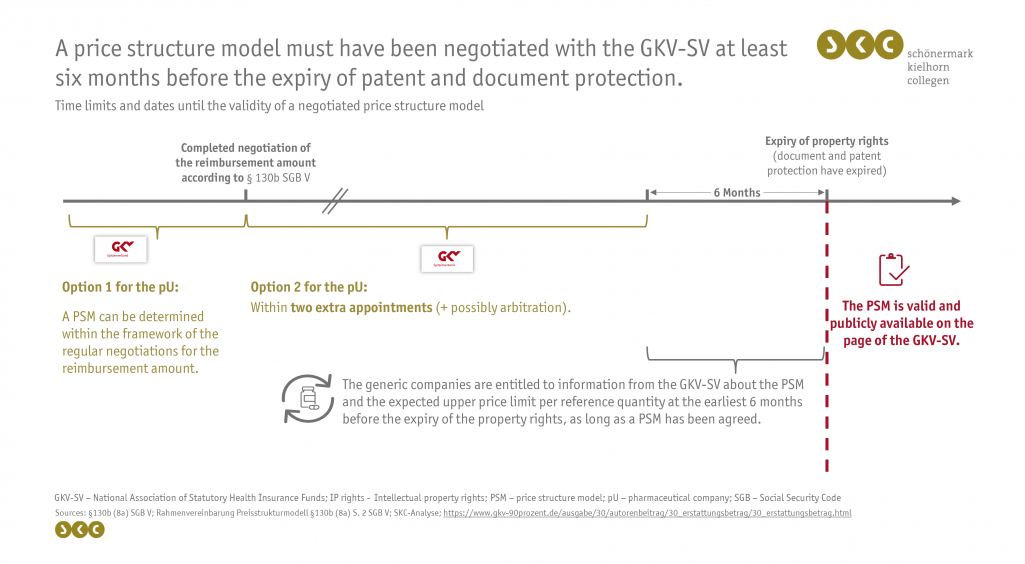
Since 1 May 2022, the PSM can be negotiated within the regular reimbursement amount negotiation or at a later stage within two additional dates. In case of non-agreement, a possible arbitration procedure follows. Contents that must be included in the PSM are the pharmaceutical central number (PZN), reference unit as well as the number of reference units of the original product and the applicable reimbursement amount per reference unit. For packs of generic medicinal products with the same quantity of medicinal products, the current reimbursement amount is the highest permissible reimbursement price. For other pack sizes, an additional formula is agreed upon, which a generic manufacturer can use to calculate the maximum permissible reimbursement price of its product. These calculations can be based on a linear (e.g. price per active ingredient quantity), a flat (e.g. price per unit of a pharmaceutical form) or a complex model (e.g. linear model up to a certain threshold value).
A PSM must have been negotiated with the GKV-SV up to six months before the expiry of the property rights, as from then on the generic manufacturers have a right to ask the GKV-SV for information about the PSM as well as the probable upper price limit per reference unit. The generic manufacturer has the right to ask for information about the PSM only once per calendar half-year. In the event that no PSM has been negotiated by this time, no further information can be given to the generic manufacturer. The GKV-SV has a right to know the duration about the property rights who must be provided by the pharmaceutical entrepreneur on the term of the active substance patent within four weeks upon request. This right to demand information does not apply to other manufacturers.
With the discontinuation of the patent and document protection, contractual aspects decided in the context of the reimbursement amount negotiations, such as the price-volume-regulation or the supersession of the mandatory manufacturer's rebate according to § 130a SGB V, no longer apply. The reimbursement amount valid at this time is the continuing maximum reimbursement amount, which cannot be adjusted upwards. Therefore, in future reimbursement amount negotiations the thematic of supersession of the mandatory rebate is strategically relevant if the highest possible continuing reimbursement amount is to be achieved after the expiry of the property rights. All pharmaceutical companies of original products who have concluded the reimbursement negotiations before April 30, 2022, have the option of adjusting the reimbursement amount by the net manufacturer rebate up to three months before the later expiry of the patent or document protection in order to have a higher maximum permissible reimbursement amount after the expiry of the patent and document protection.
With our experience in more than 80 different price negotiation procedures, we are pleased to be your competent partner for the development of a price structure model, the creation of a target-oriented negotiation strategy or the preparation and support of price negotiations.
Sources:
About the author

Consultant
M.Sc. Economics
Fax: +49 511 64 68 14 18