The "fast-track procedure" of the BfArM
the most important requirements, steps and deadlines for digital medical device manufacturers
With the "Digital Care Act" (Digitale-Versorgung-Gesetz - DVG), which came into force on December 19, 2019, a new digital service sector was established for the German statutory health insurances, enabling low-threshold access for patients to the so-called "app by prescription" and thus following a participatory approach. In this context, the Federal Institute for Drugs and Medical Devices (BfArM) was assigned the competence to conduct the review process for the nationwide approval of digital health applications (DiGA). The "Fast-Track-Assessment" is associated with important legal requirements and deadlines, whose compliance is a fundamental prerequisite for a comprehensive and compulsory reimbursement by the statutory health insurances in Germany.
In the first part of our Digital Health blog series, the general legal frameworks of the DVG and the "Digital-Health Application-Regulation" (DiGA-V) have been outlined.
In this blog, we want to give more detailed insights into the process of the "Fast-Track-Assessment", including the most recent specifications of the BfArM's DiGA guidelines.
First, it is important to differentiate which digital applications are classified as DiGA according to §33a SGB V and are therefore eligible for the "Fast-Track-Assessment" as determined in §139e SGB V. In this context, DiGA are defined as medical devices,
- which are assigned to risk class I or IIa (according to the Medical Device Directive (MDD) / Medical Device Regulation (MDR, effective from May 25, 2021 with stricter requirements for safety and quality).
- whose main function is the digital technology (and not the medical advice provided by care providers or the hardware components) and which pursue a clear medical purpose.
- which are used to diagnose, monitor, treat or alleviate diseases or disabilities (and do not serve as primary prevention)
- which are either used jointly by the patient and the care provider or solely by the patient (and thus do not function as mere "practise equipment" for the care provider).
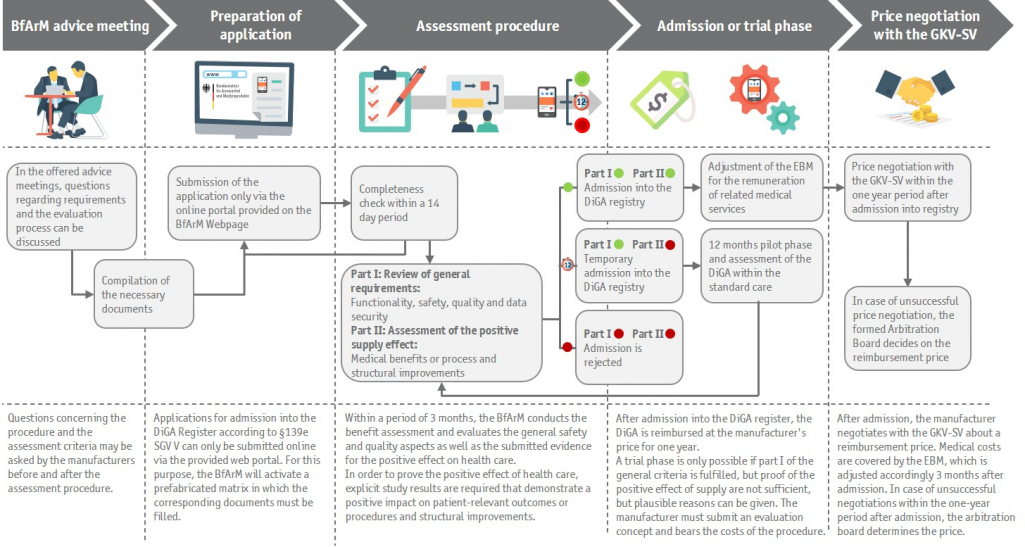
If these criteria are met, the next step can be initiated by digitally applying for the admission into the DiGA register that is managed by the BfArM. In case of uncertainties regarding the admission criteria, the BfArM provides fee-based individual consultations in which enquiries regarding the procedure as well as specific requirements for the respective digital application can be discussed. The costs must be financed by the digital medical device manufacturer and are based on the effort incurred; on this matter the BfArM has published a table of fees in their DiGA guidelines. However, advice can only be obtained before or after inclusion into the DiGA register as the BfArM-consultation during an ongoing evaluation process is not permitted.
The DiGA register will be hosted as a web portal and primarily strives to create the greatest possible transparency for health insurance companies, care providers and patients. The approval procedure for a DiGA starts with the electronic application by the digital medical device manufacturer - in compliance with the digitization premise, via a web-based application portal on the website of the BfArM, which will be activated as of mid-May. However, as of today's date (20th of May 2020) this is still pending. Subsequently, the BfArM reviews the application with respect to formal correctness within 14 days. If the formal correctness of the submission documents can be verified, the 3-month processing period for the "Fast-Track-Assessment" begins. In case of incomplete or incorrect application documents, the BfArM has the right to request the missing documents, which must then be submitted by the DiGA manufacturer within 3 months. Hence, the "Fast-Track-Assessment" only begins when the complete and formally correct documents are available for the BfArM.
During the assessment process for the digital medical device, the BfArM examines the producer's application and the corresponding documents particularly in terms of two aspects. On the one hand, the assessment of requirements for product characteristics with regard to the product safety, functionality, quality (esp. interoperability), data protection and data security, which must be evidenced by standardized checklists (annexes 1 and 2 of the DiGA-V). On the other hand, the assessment includes the examination of positive effects on health care, which must be proven by comparative studies. A positive effect on health care can be reflected in a medical benefit, e.g. in a shortening of the disease duration, as well as in measurable patient-relevant processual and structural improvements, e.g. an enhanced coordination of treatment processes or a higher adherence.
The "Fast-Track-Assessment" divides into three possible ways. In the first case, the product requirements are met and the evidence of positive effects on health care is provided by a completed comparative study. This leads to the final inclusion of the digital application into the DiGA register according to §139e SGB V. In the second case, sufficient study evidence for positive effects on health care does not yet exist, but all other product requirements have been met. This initiates the application for a preliminary admission into the DiGA register, coupled with a 12-month test phase in which the medical device manufacturer is given the opportunity to prove positive effects on health care and plausibly justify them to the BfArM by submitting a suitable evaluation concept. If positive effects on health care can be demonstrated during this period, this results in a final admission into the DiGA register within 3 months after submission of the study results to the BfArM. If it is not possible to prove positive effects on health care within these 12 month, the DiGA manufacturer can request an extension of the test phase for further 12 months in exceptional cases by submitting a plausible explanation to the BfArM, which can only be accepted if the request is made three months before the test phase is ending. Otherwise, the lack of proof leads to a final rejection of the inclusion into the DiGA register by the BfArM. In the third case, neither the product requirements have been met, nor positive supply effects could be proven, so that the medical device manufacturer receives a direct rejection notice from the BfArM. In the event of a negative decision, a new application for admission into the DiGA register is earliest possible after 12 months.
According to §134 SGB V, the first 12 months after the BfArM decision on the preliminary or final admission into the DiGA register are considered as the free pricing phase, in which the DiGA is priced at the manufacturer’s price specified in the application documents. For the free pricing phase, the GKV umbrella association (GKV-SV) and the manufacturers’ associations can set maximum prices for groups of comparable DiGA in a framework agreement. After inclusion into the DiGA register, a price negotiation takes place between the DiGA manufacturer and the GKV-SV, which culminates into a negotiated price and eventually replaces the manufacturer’s price with the beginning of the 13th month. In this context, performance-related remuneration elements such as P4P can be included in the negotiated price. In addition, the negotiated price leads to an adjustment of the Physicians' Fee Scale, the Einheitlicher Bewertungsmaßstab (EBM), and thus enables the outpatient remuneration at the expense of the German statutory health insurances. If there is no negotiation agreement between the DiGA manufacturer and the GKV-SV within the 12 months period after admission, an arbitration board will determine the reimbursement price within 3 months.
In the next article in our blog series "Digital Health Applications (DiGA) in Fast Track", we look at the challenges associated with the evaluation of the positive effect on health care and strategic issues for the application procedure.
In the course of the DVG a new service area has developed for the German health care system with a benefit assessment and reimbursement procedure, which has clear deadlines and application requirements, but also many pitfalls, which SKC can help to avoid by providing advice and operational support. In recent years, SKC has successfully completed numerous projects in the medical technology and pharmaceutical industry, thus building up profound expertise in the field of market access and reimbursement mechanisms of new drugs and medical devices. In the future, we will be available to DiGA manufacturers as a competent and reliable partner for topics related to the assessment of reimbursement eligibility, benefit assessment, and the development of price negotiation strategies for digital applications.
German Sources:
- Leitfaden „Fast-Track"-Verfahren für digitale Gesundheitsanwendungen (DiGA)
- Verordnung über das Verfahren und die Anforderungen zur Prüfung der Erstattungsfähigkeit digitaler Gesundheitsanwendungen in der gesetzlichen Krankenversicherung (DiGA-V)
- BfArM-FAQ zum Fast Track für Digitale Gesundheitsanwendungen im DVG
About the author

Founder and Managing Director
Fax: +49 511 64 68 14 18