Writing the AMNOG value dossier
SKC’s top 5 essentials
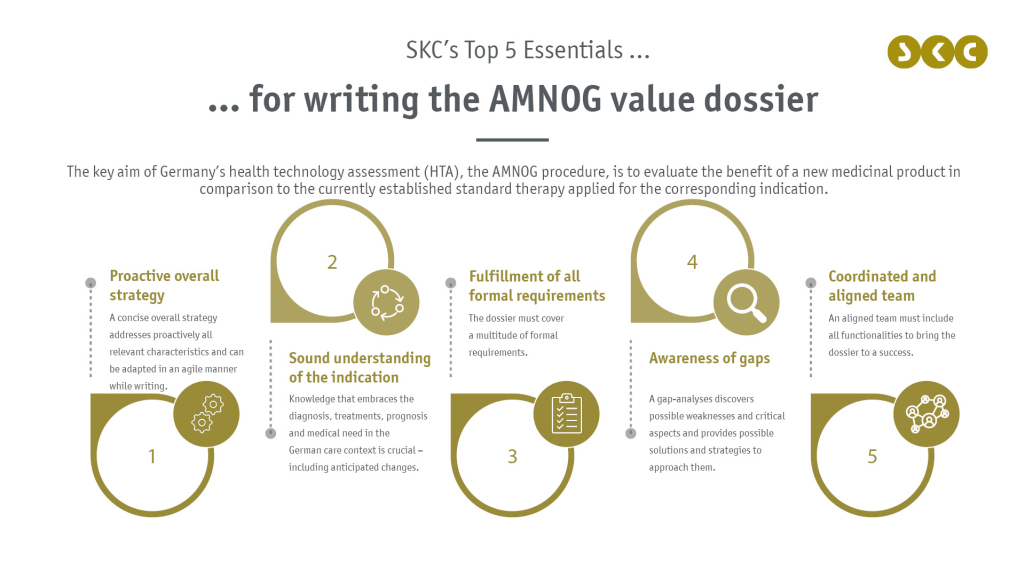
The central document that clarifies and presents all aspects required for this evaluation is the value dossier with a size most often exceeding a thousand pages in total. To stay on top of this comprehensive and yet concise document, we provide experience and knowledge coming from numerous dossiers, interactions with the German Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA), the understanding of the German HTA and health care system and a passionate team to get the best out of our client's products.
Even though the value dossier is a standardized document that must fulfill distinct requirements and follow clear regulations, its preparation is a unique process, always tailor-made for each drug and indication. To accomplish this, one must bring in a deep knowledge of the indication that covers all aspects from diagnosis over established treatments to prognosis and the medical need – all in the German care context. Beyond that, writing the dossier must be based on experience coming from various market access processes that provide an understanding of the AMNOG procedure, also incorporating relevant precedents. With SKC, our clients can benefit from our MAIS database that encompasses all previous benefit assessments and their unique features. Building on customized analyses, we can set up the required strategy to approach and cover possible weaknesses and work out the benefits of our client's product – while fulfilling the formal requirements for the AMNOG procedure.
By doing so, we at SKC make sure that all value messages of our client's products are presented with the right wording at the right places within this extensive document, in an adequate manner considering the unique German health care system and the peculiarities of the German HTA. Consequently, the process of writing the dossier is fully embedded in the entire market access process, should be thoroughly prepared and the corresponding teams should be in regular exchange to bring the project to a full success.
About the author

Market Access Manager
M.Sc. Biomedicine
Fax: +49 511 64 68 14 18